Research Article
Insects Associated with Sweetpotato, Ipomoea batatas (L.), in Mississippi [pdf]
Reed*, J. T., D. E. Fleming, T. L. Schiefer, D. Bao, and C. S. Jackson
Department of Entomology and Plant Pathology, Mississippi State University, P.O. Box 9775, Mississippi State, MS 39762-9775.
* Direct correspondence to Dr. Reed, jreed@entomology.msstate.edu
Received: 8-X-2008 Accepted: 26-1-2009
Abstract Seventy-two species or genera of insects collected from or observed in commercial sweetpotato (Ipomoea batatas [L.]) fields or research plots during a five-year period are listed. Twenty-two species are known to damage sweetpotato storage roots, 13 species are recognized as serious pests of sweetpotato, and 43 species have the potential for feeding on sweetpotato foliage. The primary root-damaging pests are beetles (Coleoptera: Chrysomelidae, Brentidae, Curculionidae, Elateridae and Scarabeidae), and noctuid moth larvae (Lepidoptera: Noctuidae). Other insects from the orders Hemiptera (8 species), Lepidoptera (9 species), Orthoptera (1 species), and Thysanoptera (3 species) are listed. Thrips, aphids, and several beetle species are discussed as possible vectors of viral diseases to sweetpotato.
Key words: sweetpotato, Ipomoea batatas, insect pests
Introduction
In Mississippi, sweetpotatoes are often planted in a rotation with cotton, corn, soybeans, pasture, or peanuts. Fields are often bordered by other row crops or mixed hardwood and pine forests. Such a diverse ecosystem provides a complex environment for the establishment of many insect species. Insect feeding might result in damage to both the foliage and the roots of sweetpotato plants, and several insects serve as vectors for viral diseases. Over 20,000 acres of sweetpotatoes were harvested in Mississippi during 2007, with a value of 67.55 million dollars (National Agricultural Statistics Service, 2008). Knowledge of the insects infesting this valuable specialty crop is important to individuals providing crop protection services or conducting research concerning insect management and disease transmission.
Insects known to damage sweetpotato storage roots during the growing season in Mississippi include representatives of several insect orders and families. The most significant pests are within the order Coleoptera. Among these are larvae of several Chrysomelid beetles (Coleoptera: Chrysomelidae), such as spotted cucumber beetle, Diabrotica undecimpuntata howardi Barber; banded cucumber beetle, D. balteata LeConte (Cuthbert and Reid 1965, Cuthbert 1967); sweetpotato flea beetle, Chaetocnema confinus Crotch (Kantack and Floyd 1956, Chalfant et al. 1979); and several flea beetles of the genus Systena (Thomas 1927, Schaulk et al. 1991). Other beetles damaging sweetpotatoes in Mississippi include two species of whitefringed beetle, Naupactus leucoloma Bohemanand N. peregrinus (Buchanan) (Coleoptera: Curculionidae) (Zehnder 1997); sugarcane beetle, Euetheola humilis rugiceps (LeConte) (Smith 2006), white grubs of the genus Phyllophaga (Coleoptera: Scarabaeidae) (Kantack and Floyd 1956, Cuthbert and Reid 1965); and wireworms primarily of the genera Conoderus, Heteroderes, and Melanotus (Coleoptera: Elateridae) (Griffin and Eden 1953, Fronk and Peterson 1956, Cuthbert 1967, Seal 1990, Chalfant and Seal 1991). The sweetpotato weevil, Cylas formicarius elegantulus (Summers), is only rarely found in Mississippi because of a highly effective quarantine imposed by the Mississippi Bureau of Plant Industry. With the exception of white grubs and sugarcane beetle, adults of these insects and numerous other insects including tortoise beetles (Coleoptera: Chysomelidae) and larvae of several moth species, primarily noctuid moths (Lepidoptera: Noctuidae), feed in the lush foliage of the sweetpotatoes. Many Lepidoptera larvae will also feed on exposed roots.
Viral diseases have long been recognized in sweetpotato and 15 viruses have been characterized (Valverde et al., 2007). Primary virus vectors include aphids (Hemiptera: Aphididae) and whiteflies (Hemiptera: Aleyrodidae). Viral diseases found in Mississippi sweetpotatoes include at least the very common sweetpotato feathery mottle virus (SPFMV), sweetpotato virus G (SPVG), and Ipomoea vein mosaic virus (IVMV) (Sabanadzovic, 2008). Sweetpotato chlorotic stunt virus (SPCSV) vectored by whiteflies in combination with the aphid-vectored SPFMV results in sweetpotato virus disease (SPVD), a particularly yield-limiting disease. Sweetpotato chlorotic stunt virus has been reported in North Carolina (Abad et al. 2007) and because the associated SPFMV and vectors are present in Mississippi, it is a potential disease for Mississippi sweetpotatoes. Although thrips (Thysanoptera: Thripidae) have not been proven to be vectors of any viral disease to sweetpotato, several species are vectors of tomato spotted leaf wilt virus to several other crops, and the potential exists for thrips to transmit disease pathogens to sweetpotato. About 30 chrysomelid species have been identified as virus vectors to various plant species, and about two thirds of those are in the subfamily Alticinae, that includes the flea beetles (Hull 2002). Many of these insects occur on Mississippi sweetpotato.
During the last several years, insects have been sampled by a variety of techniques on commercial sweetpotato production fields and research plots within the state of Mississippi. A 5-year (2004-2008) research project entitled the Southern Sweetpotato IPM Project, funded by the USDA Risk Avoidance and Mitigation Program (RAMP), was conducted to more completely document parameters associated with sweetpotato production in Alabama, Louisiana, Mississippi, and North Carolina. Insects were collected during the study with sweep-net, vacuum, sticky-card, light trap, pheromone trap, bait or by hand-collecting samples. This paper reports primarily on the sampling results of this study, but includes species collected at various other times from sweetpotato fields in Mississippi.
Materials and Methods
Although some sampling was conducted beginning in 2002, the majority of the sampling reported here occurred during the years 2004–2007. During each of these years, a strip of sweetpotatoes 8–16 rows wide (2004) or 16–32 rows wide (2005–2007) by 91.5 m long were planted in commercial sweetpotato fields without any insecticidal input. Additional treatments included strips of equivalent size with pre-plant incorporated insecticide alone, insecticide applied to the foliage only, or the grower’s protocol that included pre-plant incorporated insecticide plus foliar insecticide. Each strip plot was divided into six subplots 15.25 m long. Insects in the subplots were then sampled by making a 25-sweep sample while walking 12 m with a 38 cm (diameter) sweep-net or by vacuuming 12 m of row with a vacuum sampler. Insects were transferred from the sampling device to plastic bags and taken to the laboratory for identification and counting. After 2004, 7.6 by 12.7 cm yellow sticky cards were added to the sampling protocol for insect pests. Some sampling was accomplished by shaking the vines in 3 m of row and scanning the soil under the vines for insects. In 2004 and 2005, wireworm bait (Jansson and Lecrone, 1989) consisting of about 0.2 L of crimped oats soaked in water were placed from 5 to 15 cm in the ground in each subplot prior to planting (when possible) and again during mid-season. These baits were left in the field for 1 week, and were then dug up with a post-hole digger. The bait and soil immediately surrounding the bait were taken to the laboratory and either sifted or mixed with water to break up the soil and assist in filtering out the wireworms. The number of fields that were studied in 2004, 2005, 2006 and 2007 was 19, 19, 18, and 4, respectively, with a minimum of 12 subplots (sample sites) in each field. The total number of samples taken by sweep-net, sticky card and vacuum during the years 2004–2007 was 1684, 5050, 2375, and 767, respectively.
From 2002 to 2004, Japanese beetle pheromone traps baited with 100% isoleucine methyl ester impregnated rubber tabs were placed near sweetpotato fields in the dominant sweetpotato production areas to attract Phyllophaga ephilida (Say), the white grub most likely to be a pest in Mississippi sweetpotatoes. Although this pheromone is most attractive to P. ephilida, other species were also found in the traps. The adults collected in pheromone traps are listed, whereas grubs of the genus collected during the study were not identified.
Beneficial insects in general were not collected and counted. Coccinelid beetles (Coleoptera: Coccinellidae) and geocorid bugs (Hemiptera: Geocoridae), however, were common but not easily identified to species in the field. Specimens retained from samples during the summer were later identified and included in the listed species.
Results and Discussion
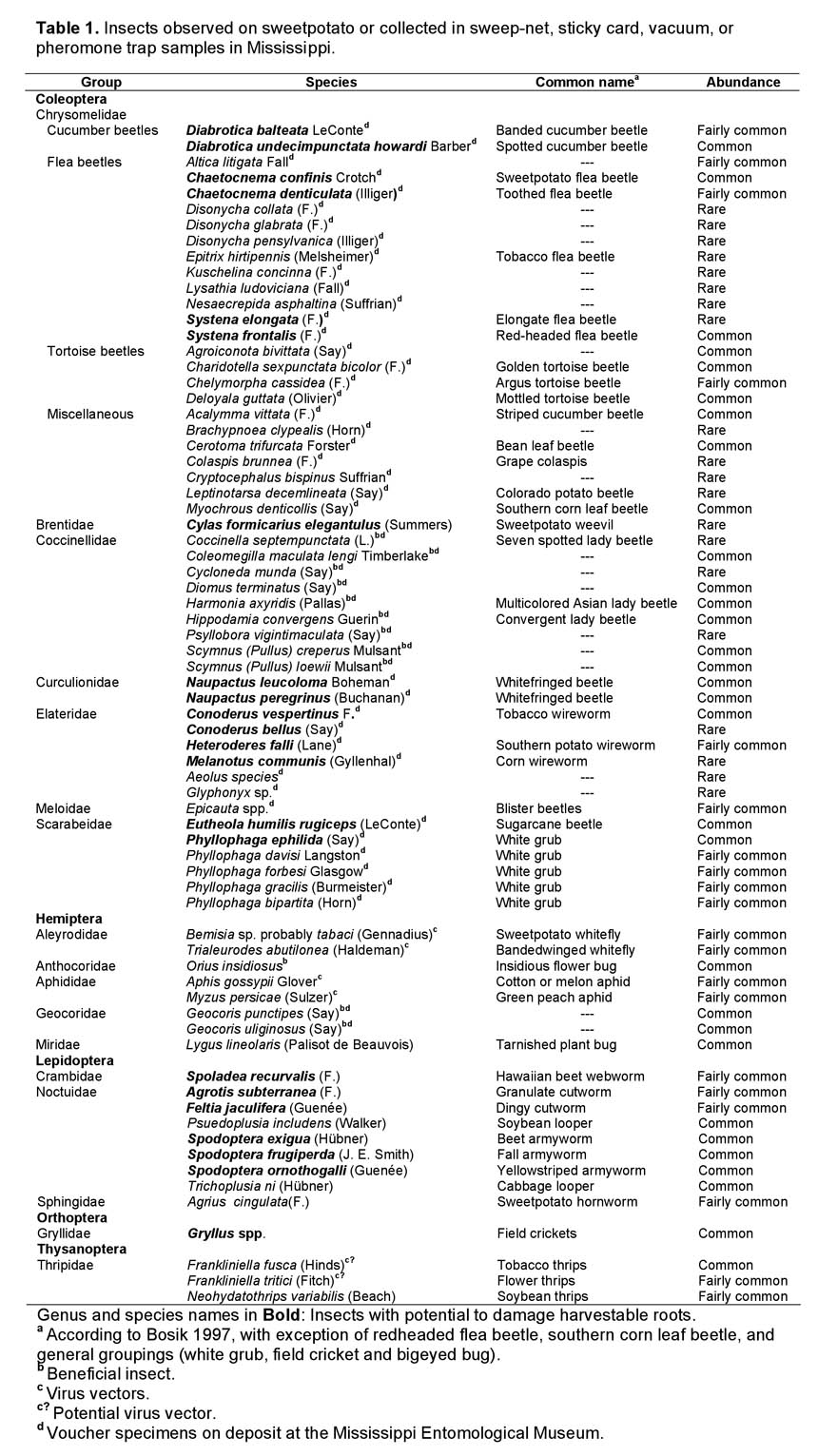
Damaging Insects. Seventy-two species or genera were identified from insects collected from commercial sweetpotato fields or research plots (Table 1). Of these, 22 are known to damage sweetpotato storage roots, and 43 species may feed on sweetpotato foliage. Thirteen species are recognized as major sweetpotato pests.
Forty potentially damaging Coleoptera species were identified in the study including six elaterid species, two curculionid species, one brentid species, 25 chrysomelid species of which 12 were collected rarely, and six scarabaeid species. The most frequently collected wireworm adult was Conoderus vespertinus F.Special note should be made concerning the corn wireworm, Melanotus communis (Gyllenhal).Only one specimen of adult corn wireworm (species identification not verified) was collected in thousands of insect samples from fields in Mississippi from 2003–2007.Larvae collected from one field that was abandoned because of severe wireworm infestation were identified as a Melanotus species, possibly M. communis, indicating the obvious potential of the pest within the state. It is a major pest of sweetpotatoes in North Carolina, but has been rarely reported from Mississippi. With the growing acreage of corn in the state, this pest may become more prominent. The two weevils, Naupactus leucoloma and N. peregrinus may occur in such high numbers in Mississippi as to damage nearly 100% of potatoes within single fields. The sweetpotato weevil, Cylas formicarius elegantulus (Summers), is rarely found in Mississippi since a quarantine by the Mississippi Bureau of Plant Industry protects the sweetpotato growing areas of the state. Phyllophaga ephilida, a recognized pest of sweetpotato,was collected in high numbers in pheromone traps. It is assumed that P. ephilida is the primary grub pest of sweetpotato in Mississippi, but the actual number of species feeding on sweetpotato roots in Mississippi is still conjectural.
Many insects feed on roots that are exposed by soil erosion or cracking of the soil late in the season. Cutworms and several species of armyworms may be present in high numbers in fields when vines are destroyed in preparation for harvest. In absence of foliage, larvae move into cracks in the soil caused by expanding sweetpotato roots or drying soil and feed on storage roots. In addition to the Lepidoptera, crickets of the genus Gryllus (Orthoptera: Gryllidae) were observed in sweetpotato fields in Mississippi and will feed on exposed sweetpotatoes. Other Orthoptera, grasshoppers and katydids, were commonly seen on sweetpotato foliage, but were not observed feeding on exposed storage roots.
The two aphid species identified from sweetpotato in this study, Aphis gossypii Glover and Myzus persicae (Sulzer) (Hemiptera: Aphididae), are both recognized vectors of viral disease to sweetpotato (Vasquez et al. 2008). Two whiteflies, Bemisia sp., possibly B. tabaci (Gennadius) or B. argentifolii (previously B. tabaci biotype B) and Trialeurodes abutilonea (Haldeman) (Hemiptera: Aleyrodidae), may be present in sweetpotatoes in Mississippi, but are generally in very low numbers. Whiteflies and aphids are vectors of viral diseases to sweetpotato that include sweetpotato chlorotic stunt virus (SPCSV) and sweetpotato feathery mottle virus (SPFMV). Two of the thrips species, Frankliniella tritici (Fitch) and Frankliniella fusca (Hinds) (Thysanoptera: Thripidae), collected on sticky cards in sweetpotato fields are known vectors of tomato spotted leaf wilt virus (TSWV) in crops other than sweetpotato. These species have not been shown to be vectors of any viral disease to sweetpotato, but, since TSWV has been isolated from sweetpotato (Valverde et al. 2007), there exists the potential for them to transmit this or other diseases to sweetpotato as well. Nine species of alticine flea beetles were identified from sweetpotatoes in Mississippi, and some of these may be vectors of viral diseases. Spotted cucumber beetleand Ceratoma trifurcata (Forster), the bean leaf beetle,have also been identified as virus vectors in other host plant systems (Wang et al. 1994a, b). It is possible that several of the 25 chrysomelid species found on sweetpotato in this study could be added to the potential vector list. Thus many of the insects found in sweetpotato may be able to diminish yield, quality, or both by physical damage and by disease transmission.
Beneficial Insects. Nine coccinellid species (Coleoptera: Coccinelidae) were identified from insects collected from sweetpotato foliage. These are well known as predators of aphids. In Mississippi, aphids are present in very low numbers in sweetpotato, possibly because they are not well adapted to sweetpotatoes, and perhaps because of continual presence of several species of lady beetles in relatively high numbers in sweetpotato foliage.
Geocoris punctipes (Say) and G.uliginosus (Say) (Hemiptera: Geocoridae) were common in insect samples from sweetpotato throughout the growing season. Orius insidiousus (Say) (Hemiptera: Anthocoridae) was also commonly present in sweetpotato fields. Several species of nabids (Hemiptera: Nabidae) were also observed in the field, but were not collected and identified.
Many other species of insects were observed in sweetpotato fields. Fire ants were plentiful late in the season, and numerous species of ground beetles (Coleoptera: Carabidae), including tiger beetles, were present under the leaf canopy. These insects in conjunction with many species of spiders serve as a formidable beneficial arthropod group to help suppress Lepidoptera larvae and other insects within and beneath the sweetpotato canopy.
Comments. As listed in Table 1, the abundance of insects in the field (common, fairly common, and rare) serve only as general indicators of frequency of collection or observation. Insects that occur sporadically in high numbers in the field, those that occur at consistently low levels, or those that occur annually for a short period of time during the season may all be considered fairly common. The percentage of sweep-net samples in which 1 or more of the listed insects or insect groups were collected during early, mid- and late season helps to demonstrate this commonality (Table 2). All of these insects are considered common in sweetpotato fields, even though they are represented by vastly different numbers in sweep-net collections.
There is a general lack of knowledge concerning beneficial insects in production sweetpotato fields and their value, and much remains to be determined about the pest status of many insects. The identification of both beneficial and pest insects is essential for the interpretation of predator-prey relationships and for the development of integrated pest management criteria. Further identification of the insect complex in sweetpotatoes is needed to ascertain value or risk of each species and incorporate such knowledge in pest management recommendations. This paper serves as a contribution to the science of integrated pest management by providing a list of insects associated with the sweetpotato crop in Mississippi.
Acknowledgements
We acknowledge the following individuals for invaluable assistance in identification of species: Ed Riley, Texas A & M University (chrysomelid beetles); Rebecca Baumler, North Carolina State University (elaterid larvae); Blake Layton, Mississippi State University (aphids, whiteflies and Lepidoptera larvae); Richard Brown, Mississippi State University (Lepidoptera larvae); and David Willers, Texas A & M University (Phyllophaga species).
References
Abad, J. A., E. J. Parks, S. L. New, S. Fuentes, W. Jester, and J. W. Moyer. 2007. First report of sweetpotato chlorotic stunt virus, a component of sweetpotato virus disease, in North Carolina. Plant Disease 91: 327.
Bosik, J. J. 1997. Common names of insects and related organisms. Entomological Society of America. Lanham, Maryland. 232 pp
Chalfant, R. B., and D. R. Seal. 1991. Biology and management of wireworms on sweet potato, pp. 303-326. In R.K. Jansson and K.V. Raman [eds.], Sweet Potato Pest Management: A Global Perspective. Westview Press, Boulder, Colorado.
Chalfant, R. B., S. A. Harmon, and L. Stacey. 1979. Chemical control of the sweet potato flea beetle and southern potato wireworm on sweet potatoes in Georgia. Journal of Georgia Entomological Society 14: 354-358.
Cuthbert, F. P. 1967. Insects affecting sweetpotatoes. Agricultural Handbook, No. 239. Agricultural Research Service, United States Department of Agriculture. 28 pp.
Cuthbert, F. P. Jr. and W. J. J. Reid. 1965. Four little-known pests of sweetpotato roots. Journal of Economic Entomology 58: 581-583.
Fronk, W. D. and L. E. Peterson. 1956. Wireworm control in Iowa sweetpotato fields. Journal of Economic Entomology 49: 479-481.
Griffin, J. A., and W. G. Eden. 1953. Control of the gulf wireworm in sweetpotatoes in Alabama. Journal of Economic Entomology 46: 948-951.
Hull, R. 2002. Mathew's Plant Virology, 4th Edition. Academic Press, New York, New York. 1001 pp.
Jansson, R. K., and S. H. Lecrone. 1989. Evaluation of food baits for pre-plant sampling of wireworms (Coleoptera: Elateridae) in potato fields of Southern Florida. Florida Entomologist 72(3): 503-510.
Kantack, E. J., and E. H. Floyd. 1956. Control of some insects which damage roots of sweet potatoes in the field. Journal of Economic Entomology 49: 766-768.
National Agricultural Statistics Service. 2008. United States Department of Agriculture, Quick Stats. Agricultural Statistics Data Base. (http://www.nass.usda.gov/). Accessed 20 Aug. 2008.
Sabanadzovic, S. 2008. Personal communication, Aug. 1, 2008. S. Sabanadzovic, Associate Professor, Department of Entomology and Plant Pathology, P.O. Box 9775, Mississippi State University, Mississippi State, MS 39762. Phone (662) 325-2085; ssabanadzovic@entomology. msstate.edu.
Schaulk, J. M., A. Jones, P. D. Dukes, and J. K. Peterson. 1991. Approaches to the control of multiple insect problems in sweet potato in the southern United States, pp. 283-301. In R. K. Jansson and K. V. Raman [eds.], Sweet Potato Pest Management: A Global Perspective. Westview Press, Boulder, Colorado. 458 pp.
Seal, D. R. 1990. The biology of wireworms affecting sweet potatoes in Georgia. PhD Dissertation, University of Georgia, Athens Georgia. 180 pp.
Smith, T. P. 2006. Biology and chemical ecology of sugarcane beetle and integrated pest management of sweet potato soil insects in Louisiana. PhD Dissertation, Louisiana State University and Agricultural and Mechanical College, Baton Rouge, Louisiana. 110 pp.
Thomas, W. A. 1927. Injury to sweet potato by Systena taeniata var. blanda larva. Journal of Economic Entomology 20: 236-237.
Valverde, R. A., C. A. Clark, and J. P. T. Valkonen. 2007. Viruses and virus disease complexes of sweetpotato. Plant Viruses 1(1):116-126.
Vasquez, E., V. Amante, and J. O'Sullivan. 2008. Aphids. Sweet Potato Diagnostics. On line posting (http://www.lucidcentral.com/keys/sweetpotato/key/Sweetpotato%20Diagnotes/Media/Html/TheProblems/Pest-SuckingInsects/Aphids/Aphids.htm). Accessed 20 Aug. 2008.
Wang, R. Y., R. C. Gergerich, and K. S. Kim. 1994a. The relationship between feeding and virus retention time in beetle transmission of plant viruses. Phytopathology 84: 995-998.
Wang, R. Y., R. C. Gergerich, and K. S. Kim. 1994b. Entry of ingested plant viruses into the hemocoel of the beetle vector Diabrotica undecimpunctata howardi. Phytopathology 84: 147-153.
Zehnder, G. 1997. Population dynamics of whitefringed beetle (Coleoptera: Curculionidae) on sweet potato in Alabama. Environmental Entomology 26: 727-735.