Research Article
Examination of the Internal Morphology of the Ixodid Tick, Amblyomma maculatum Koch, (Acari: Ixodidae); a “How-to” Pictorial Dissection Guide [pdf]
Edwards, K. T.1* J. Goddard1, and A. S. Varela-Stokes2.
1Department of Entomology & Plant Pathology, Mississippi State University, Mississippi State, MS, 2Department of Basic Sciences, Mississippi State University, Mississippi State, MS.
Received: 24-XI-2008 Accepted: 21-I-2009
Keywords Amblyomma maculatum, Gulf Coast tick, dissection, methods
Introduction
There is copious scientific literature on the anatomical structure of ixodid ticks (Acari: Ixodidae), as well as descriptions of the physiology (Obenchain and Galun 1982, Sonenshine 1991, Buczek 1999) of their digestive tracts and diagrams of their internal anatomy (Douglas 1943, Obenchain and Galun 1982). There are also stunning scanning electron microscopy images of tick mouthparts and other structures that are readily available on the Internet (Balashov Iu and Raikhel 1973, (Pickering 2008)). However, there is inadequate documentation of how to dissect a tick (Balashov 1968, Obenchain and Galun 1982), a process required for many tick and disease studies (Goddard and Norment 1986, Goddard 2003). The literature contains numerous line drawings (Balashov 1968, Obenchain and Galun 1982) and instructions describing the process, but none is adequate for the novice tick dissector. One possible exception is found on a web page hosted by the Iowa State University Department of Entomology, where a limited midgut dissection sequence is presented for Ixodes scapularis Say (VanDyk 2001). However, for the most part, unless one has the guidance of an individual who is both knowledgeable and skilled in tick dissection, it is extremely difficult to teach oneself the complete tick dissection process from currently available materials.
Since there is little assistance in the literature on how to dissect ticks, and specifically, how to identify structures at each step of the dissection, our tutorial is intended as a stand-alone document to guide an individual who is new to the process through the necessary steps. We offer a “how-to” description and pictorial essay outlining our approach to tick dissection. The ticks used in this study were adult male and female Gulf Coast ticks, Amblyomma maculatum Koch, though the methods described herein could be applied to any species. None of the A. maculatum specimens we dissected adequately demonstrated ovaries, so we used one female A. americanum L. tick that we found to show them more clearly. The tissues we targeted are those we were analyzing for our research and are commonly dissected. Specifically, we included salivary glands, midgut, Malpighian tubules and ovaries in this study (Figure 1). Tracheae are also delineated because they might easily be confused with Malpighian tubules.
Approach
The following is a step-by-step pictorial description of the dissection of the Gulf Coast tick, A. maculatum. Note: since field-caught ticks are potentially infected with a variety of disease agents, a few precautions might be necessary. For example, a white board with double-sided sticky tape around the edges may be used to sort ticks, and gloves should be used during dissection since the infectious status of ticks is often unknown. In addition, aerosolization of tick fluids is possible as tick parts are mixed with saline and should be avoided by wearing a mask during the dissection process. All ticks, parts, fluids, and supplies should be disposed of in clearly marked biohazard bags after completion of tick dissections. The dissection process is described in the following steps, shown pictorially following the text:
- Prepare a small Petri dish ahead of time by pouring melted paraffin into the bottom and allowing it to cool and to solidify.
- Briefly heat the center of the small paraffin-filled Petri dish. (We found that a simple approach was to use the tip of a glue gun to melt the paraffin, but a heated spoon might also suffice.)
- Once the paraffin is warm and starts to melt, gently grasp the tick with forceps, pressing its legs and ventrum into the heated paraffin thereby immobilizing it and restraining its legs.
- Cover the tick with a drop of phosphate buffered saline (PBS). This is an important step because it prevents desiccation of the tissues.
- Remove the scutum with a microscalpel by first cutting across the dorsal shield at the most anterior point, just distal to the basis capitulum. (A regular scalpel is much too large and we therefore recommend using a microscalpel.)
- Continue cutting around the edge of the scutum, inserting the microscalpel into the groove just inside the festoons. Lift the dorsal exoskeleton by using forceps held in one hand while carefully dissecting the attached muscles and connective tissue with the microscalpel in the other hand. Cut the dorsum and remove completely.
- At this point, connective tissue and tracheae are apparent and usually have to be removed in order to observe deeper structures.
- Observe the anterior salivary glands. These are clear, grape-like structures at the proximal end of the tick. There are also other sets of salivary glands located near the midgut.
- Observe the gut. The gut is a dark red, spider-shaped structure.
- Tracheae can be seen originating from spiracular plates and must be differentiated from Malpighian tubules.
- Observe the Malpighian tubules. These tubules are clear, thin, tube-shaped structures that often contain urea, which appears white within the tubules. Connective tissue or tracheae might also appear white, but these are flat and wispy rather than tube shaped. Further distinction between tracheae and connective tissue is often challenging. Tracheae originate from spiracular plates but connective tissue often lacks obvious organization.
- Observe the ovaries. These appear as an inverted U-shaped structure distal to the rectal sac.
Please note: All figures refer to Amblyomma maculatum Koch ticks unless otherwise stated.
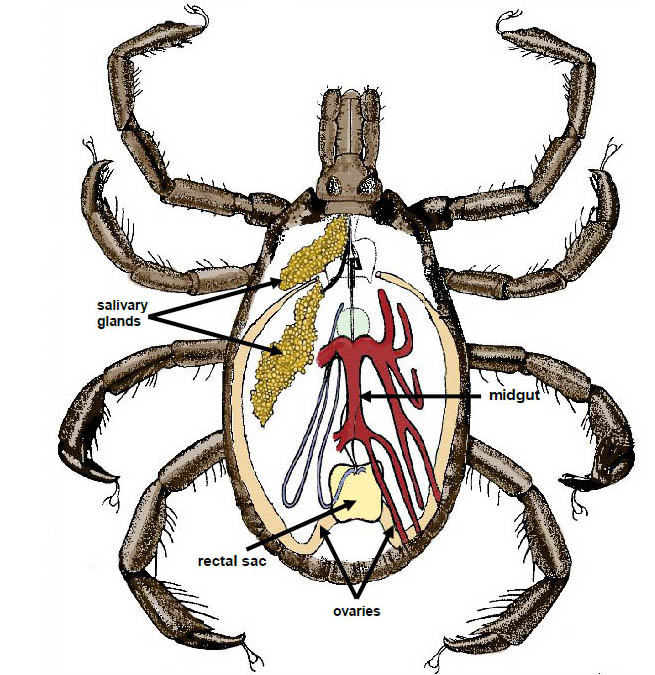
Figure 1. Line drawing of structures that may be observed during dissection (Original art work by Sylvia Burnett, Mississippi Department of Health; modified by senior author).
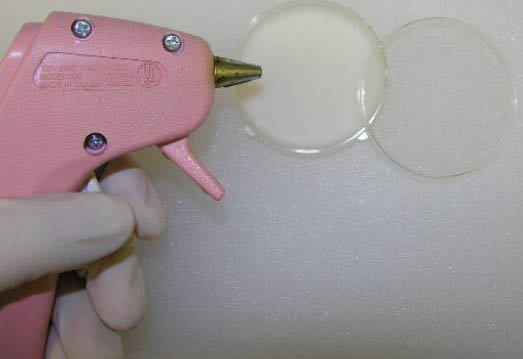
Steps 1 and 2: Using glue gun to melt paraffin in Petri dish.

Step 3. Tick imbedded in paraffin.

Step 4. Female embedded in paraffin, covered with PBS; dorsal view.

Step 5. Male embedded in paraffin; dorsal view. Preparing to remove scutum (bracket) with microscalpel starting at basis capitulum (arrow).
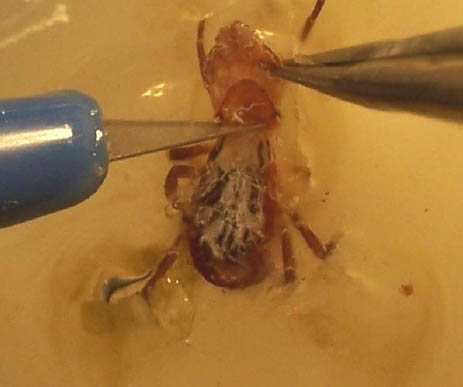
Step 6. Female; scutum removal.

Step 6 (cont.). Female; scutum removed (arrow).
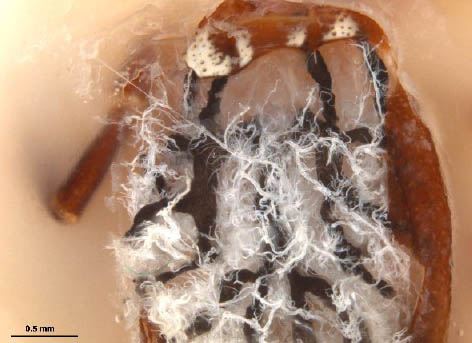
Step 7. Female; scutum removed. Connective tissue and tracheae (Philip and White 1955) are visible in contrast with the surrounding gut (dark).

Step 8. Female; scutum removed. The complete gut is now visible, as are the anterior salivary glands (arrow).
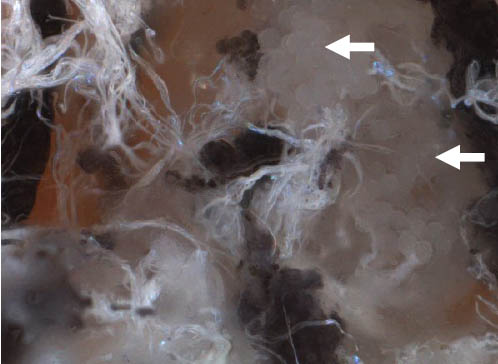
Step 8 (cont.). Salivary glands (arrow); close-up.
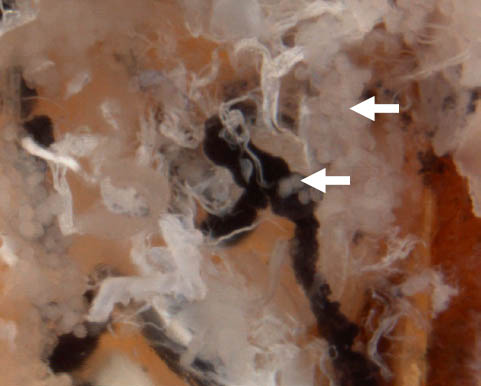
Step 8 (cont.). Female; close-up of salivary glands (arrows) near midgut.

Step 9. Female; gut visible as dark, red, spider-shaped structure (arrows).
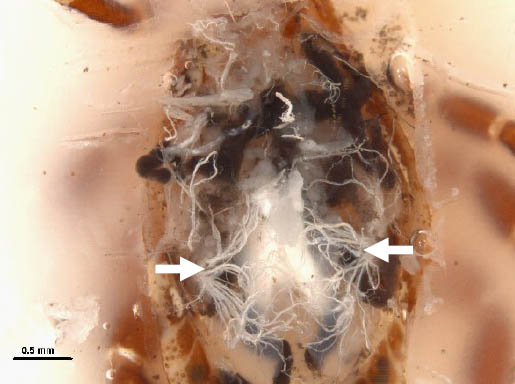
Step 10. Male; tracheae (arrows) originating from spiracular plates.
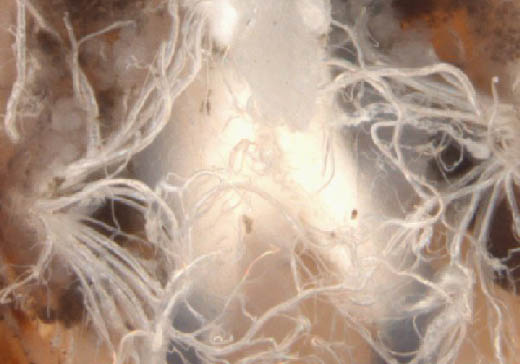
Step 10 (cont.). Male; tracheae close-up.

Step 11. Male; Malpighian tubules (curved arrow), rectal sac (white arrow), and midgut (black arrow).
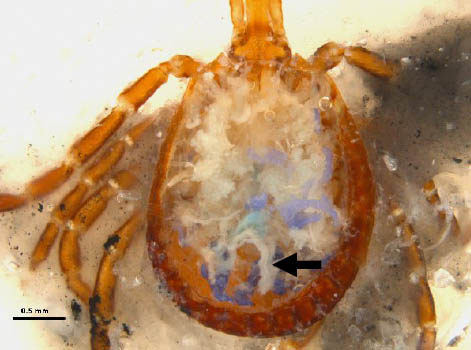
Step 12. Gut removed and ovaries (arrow) in a female A. americanum tick.
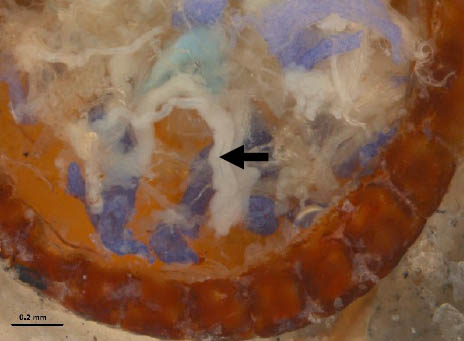
Step 12 (cont.). Close-up of previous tick with gut removed to reveal ovaries (arrow) in a female A. americanum tick.
Acknowledgements
We gratefully acknowledge Joe MacGown, Department of Entomology & Plant Pathology, Mississippi State University for his assistance in taking photographs 4-12 and to Erle Chenney, Department of Basic Sciences, Mississippi State University for his assistance in preparing the Petri dishes. Funding was provided by the Department of Entomology & Plant Pathology and the College of Veterinary Medicine, Mississippi State University. This article has been approved for publication as Journal Article No. J-11498 of the Mississippi Agriculture and Forestry Experiment Station, Mississippi State University.
References
Balashov Iu, S., and A. S. Raikhel. 1973. Electron microscopic study of the Malpighian tubules of the tick Hyalomma asiaticum (Schulze and Schlottke)empty females. Parazitologiia 7: 231-237.
Balashov, Y. S. 1968. Bloodsucking Ticks (Ixodoidea)—Vectors of Diseases of Man and Animals. Medical Zoology Department, United States Naval Reserve Unit Number Three, Cairo, Egypt.
Buczek, A. 1999. Physiology of ticks (Ixodida) in non-parasitic phase of life cycle. Wiad Parazytol 45: 151-159.
Douglas, J. 1943. Anatomy of Dermacentor andersoni Stiles. University of California Press, Berkeley and Los Angeles.
Goddard, J. 2003. Experimental infection of Lone Star ticks, Amblyomma americanum (L.), with Rickettsia parkeri and exposure of Guinea pigs to the agent. 40: 686-689.
Goddard, J., and B. R. Norment. 1986. Spotted fever group rickettsiae in the lone star tick, Amblyomma americanum (Acari: Ixodidae). Journal of Medical Entomology 23: 465-472.
Obenchain, F. D., and R. Galun. 1982. Physiology of Ticks. Pergamon Press. Oxford, Great Britian.
Philip, C. B., and J. S. White. 1955. Disease agents recovered incidental to a tick survey of the Mississippi Gulf Coast. Journal of Economic Entomology 48: 396-399.
Pickering, J. 2008. Discover Life. Texas A&M University. On-line posting (http://pick4.pick.uga.edu/mp/20q?search=Amblyomma+maculatum&guide=Arachnida), accessed 14 Nov, 2008.
Sonenshine, D. E. 1991. Biology of Ticks. Oxford University Press, New York.
VanDyk, J. 2001. Ixodes scapularis tick dissection. Iowa State University, Department of Entomology, Ames, Iowa, http://www.ent.iastate.edu/imagegal/ticks/iscap/tickdissection/entiresequence.html, accessed 18 Nov, 2008.
