Program and Abstracts of the 56th Annual Conference of the Mississippi Entomological Association, October 27–28 2009 [pdf]
Program:
![]()
![]()
8:45 How and why we train insects: Pavlovian conditioning and beyond. Dr. Glen Rains, Agricultural Technologist, University of Georgia.
9:25 Managing the economic impact of horn flies and stable flies on cattle production. Dr. Lane Foil, Veterinary Entomologist, Louisiana State University.
10:05 Break
10:20 Natural products for insect management: ARS research in Oxford, MS. Dr. Steven Duke, Natural Products Utilization Research Laboratory, USDA-ARS.
11:00 MEA Business Meeting
12:00 Awards Luncheon
![]()
Concurrent session A
1:30 Spotted cucumber beetle: pest of sweetpotato????? J. T. Reed, D. Bao and D. Fleming. Dept. of Entomology and Plant Pathology, Mississippi State University
1:42 Update on the USDA-ARS SIMRU Bt resistance research program. C. A. Blanco. USDA-ARS Southern Insect Management Research Unit, Stoneville, MS
1:54 Insect management in Mississippi rice. J. Gore and D. Cook. Delta Research and Extension Center, Mississippi State University, Stoneville, MS
2:06 Organophosphate resistance in the tarnished plant bug: population variation in major detoxification enzyme activity and gene expression. Yu Cheng Zhu. USDA-ARS Jamie Whitten Delta States Research Center, Stoneville, MS.
Concurrent session B
1:30 Efficacy of fire ant mound treatments. M.B. Layton, J. McAdory, and H. Hudson. Dept. of Entomology and Plant Pathology, Mississippi State University
1:42 Caterpillars as Taxonomists—Host plant preferences of lepidoptera supporting the phylogenetic classification of angiosperms. R. L. Brown. Dept. of Entomology and Plant Pathology, Mississippi State University
1:54 Ecological studies of the Gulf Coast tick, Amblyomma maculatum. J. Goddard and A. S. Varela-Stokes. Dept. of Entomology and Plant Pathology, Mississippi State University; College of Veterinary Medicine, Dept. of Basic Sciences, Mississippi State University.
2:06 A Basic Design for Precision Agricultural Experiments. J. L. Willers, G. A. Milliken and J. N. Jenkins. USDA-ARS, Mississippi State, MS
Student Competition
2:18 Monitoring three cornered alfalfa hoppers in early soybean productions systems. I. Pulakkatu Thodi, F. R. Musser, J. Gore. Dept. of Entomology and Plant Pathology, Mississippi State University
2:30 Landscape dynamics of tarnished plant bugs. A. Kumar and F.R. Musser. Dept. of Entomology and Plant Pathology, Mississippi State University
2:42 Effect of insecticide seed treatments on twospotted spidermite in cotton. J. F. Smith, A. L. Catchot and F.R. Musser. Dept. of Entomology and Plant Pathology, Mississippi State University
2:54: Break
3:18 Determining cotton bollworm thresholds based on white flower injury at five infestation levels in dual gene cotton. B. Von Kanel and G.M. Lorenz. Dept. of Entomology, University of Arkansas; University of Arkansas Cooperative Extension Service.
3:30 Yield lost effects from simulated insect defoliation on soybeans. L. N. Owen, A.L. Catchot, F. R. Musser, J. Gore and D. R. Cook. Dept. of Entomology and Plant Pathology, Mississippi State University; Delta Research and Extension Center, Mississippi State University, Stoneville, MS
3:42 Rickettsia parkeri in Gulf Coast Ticks (Amblyomma maculatum) infesting Mississippi Cattle. K. T. Edwards, J. Goddard, and A. Varela-Stokes. Dept. of Entomology and Plant Pathology, Mississippi State University; College of Veterinary Medicine, Dept. of Basic Sciences, Mississippi State University.
3:54 Detection of Rickettsia parkeri in the Gulf Coast tick, Amblyomma maculatum Koch, in Mississippi. F. Girao, A. Varela-Stokes, J. Goddard. College of Veterinary Medicine, Dept. of Basic Sciences, Mississippi State University; Dept. of Entomology and Plant Pathology, Mississippi State University
4:06 Phylogenetic relationships of the New World and Australian Schoenobiinae. E. L. Martinez and R.L. Brown. Dept. of Entomology and Plant Pathology, Mississippi State University.
4:18 Molecular Characterization of Popillia japonica midgut proteins. N. Syed, S. Alm and S. Karim. Dept. of Biological Sciences, The University of Southern Mississippi.
4:30 Defining the role of glutaminyl cyclase in tick physiology. H. Hammond and S. Karim. Dept. of Biological Sciences, The University of Southern Mississippi.
4:42 Exploring the Gopher Tortoise tick (Amblyomma tuberculatum) salivary gland transcriptome. P. Singh and S. Karim. Dept. of Biological Sciences, The University of Southern Mississippi.
4:54 The lone star tick, Amblyomma americanum (Acari: Ixodidae) as a vector of tick-borne agents in Mississippi. A.H. Castellaw, J. Showers, J. Goddard, E.F. Chenney, A.S. Varela-Stokes. College of Veterinary Medicine, Dept. of Basic Sciences, Mississippi State University; Dept. of Entomology and Plant Pathology, Mississippi State University.
5:06 The role of small animals in the natural history of Rickettsia parkeri. G. Mararu, J. Goddard, A.S. Varela-Stokes. College of Veterinary Medicine, Dept. of Basic Sciences, Mississippi State University; Dept. of Entomology and Plant Pathology, Mississippi State University.

![]()
Open discussions concerning all insect pests of cotton, corn, soybeans, sorghum, rice and other crops.
Extension and research entomologists, representatives from prominent farm chemical companies, and consultants will be present to delineate problems and suggest solutions.
![]()
Of particular interest to veterinarians and veterinary medical students, this round table will address insects and ticks of veterinary and medical importance. It will feature medical and veterinary entomologists, veterinary parasitologists, research scientists, extension entomologists, and industry representatives. Please bring your questions for the experts regarding arthropods of veterinary and medical importance and be prepared to enjoy a lively discussion of arthropod issues every veterinarian encounters.
Received Abstracts:
Spotted cucumber beetle: pest of sweetpotato? J. T. Reed, D. Bao and D. Fleming. Dept. of Entomology and Plant Pathology, Mississippi State University.
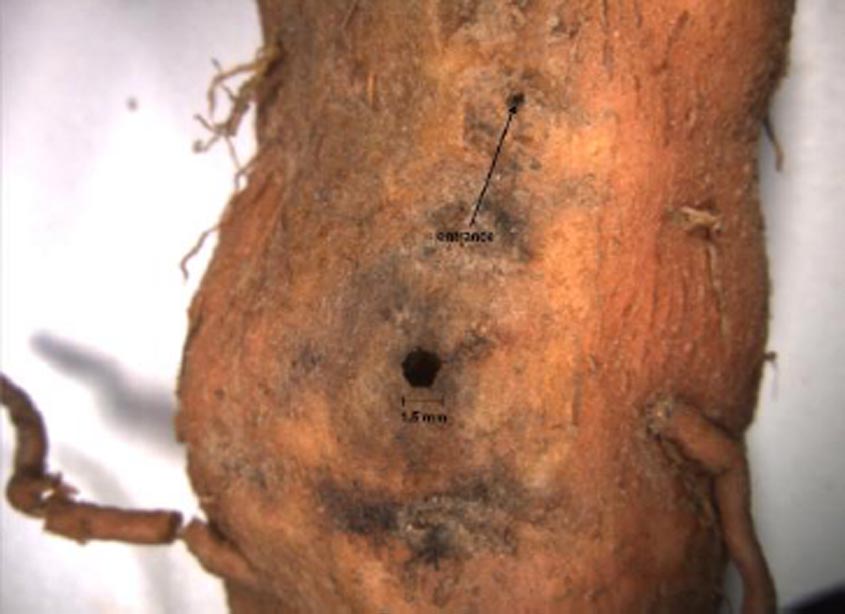
The spotted cucumber beetle (SCB), Diabrotica undecimpunctata howardi Barber, has been reported as a pest of sweetpotato for many years. However, its potential as a damaging pest of sweetpotatoes has been clouded by the fact that D. balteata, the banded cucumber beetle, wireworms, or Systena flea beetles, all capable of producing similar damage to sweetpotatoes, have been nondifferentially listed with SCB in published pesticide evaluation results for many years. Banded cucumber beetles are relatively rare in the sweetpotato growing area of Mississippi, but spotted cucumber beetles are common season-long. In addition, there appear to be few predators and parasites of the SCB in sweetpotato fields, and no known pathogens were found in field collected adults examined for pathogens. These facts, in addition to high fecundity of about 500 eggs or more per female, the ability to develop on sweetpotato roots and cause damage (Fig. 1), and development of several generations per year, suggest great economic pest potential. However, results of a recent 4-year study of insect damage of sweetpotatoes in commercial sweetpotato fields failed to correlate numbers of SCB adults in insect samples to insect damaged sweetpotatoes at harvest (Fig. 2), and field cages with multiple paired adults failed to produce expected damage to developing sweetpotatoes. The question is: why have we failed to verify economic importance?
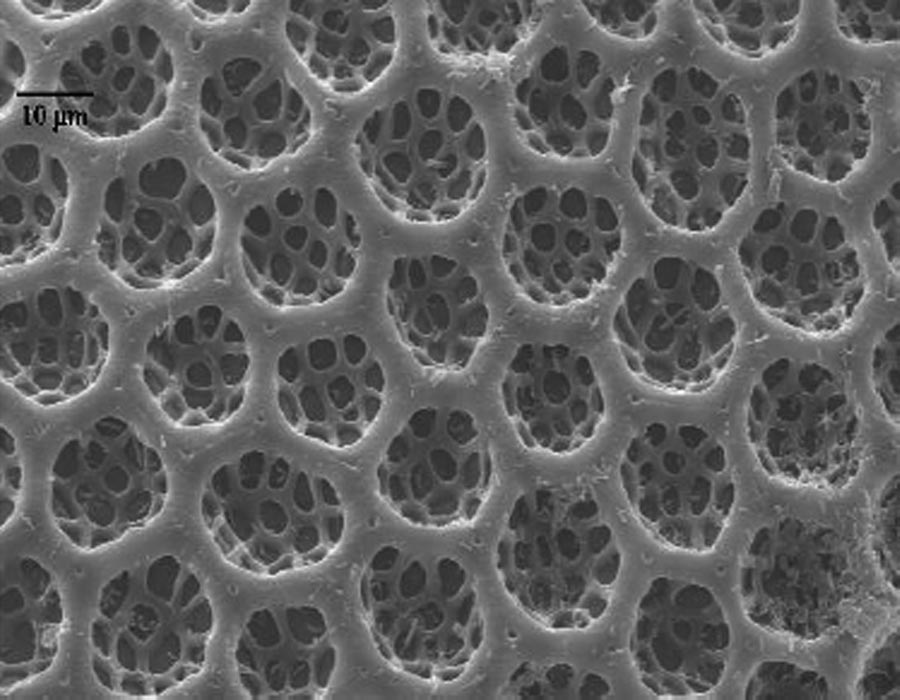
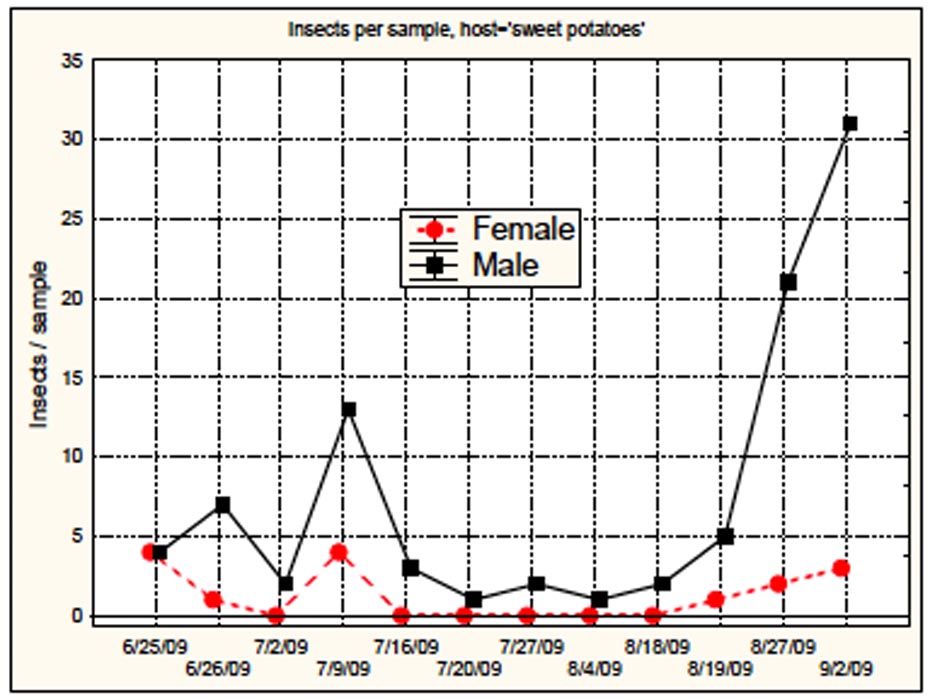
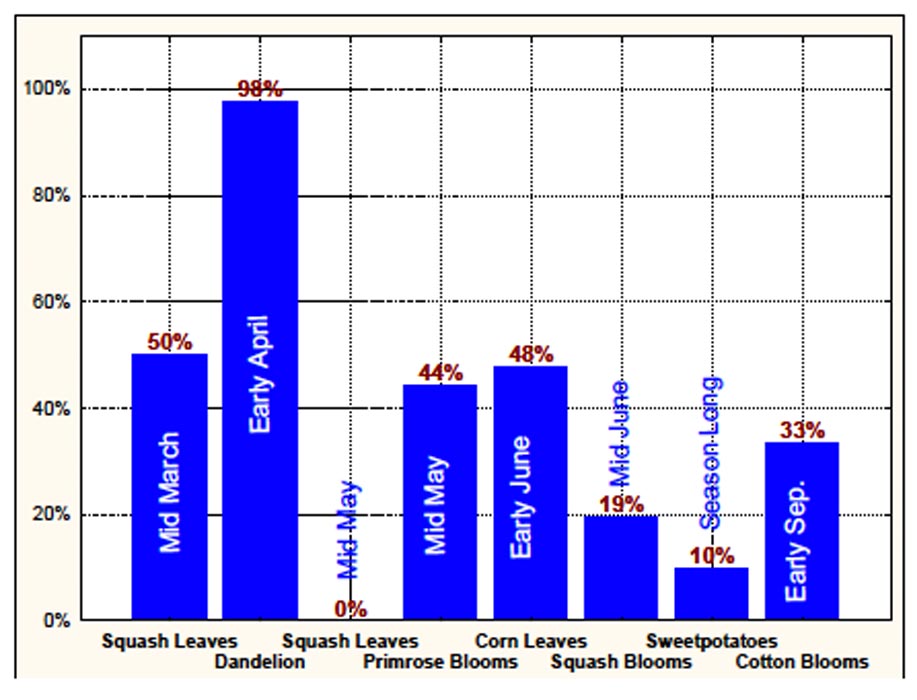
Several factors reduce the potential for SCB to be successful in sweetpotato fields. Pollen, needed by females for viable egg production, is lacking in weed-free sweetpotato fields. Eggs possess hexagonal cells (Fig. 3) which trap air that helps prevent drowning. They require nearly 100% humidity to hatch and are adapted to wet conditions usually absent in the Mississippi sweetpotato growing belt. Finally, the male/female ratio of SCB adults in sweetpotato fields in late August of 2008 (10 males/1 female) and most of the growing season during 2009 (Fig. 4) suggests that females avoid sweetpotato fields. Male/female ratios of the insect on other crops tend to support this hypothesis (Fig. 5). We hypothesize that females foraging for sources of pollen either leave sweetpotato fields or are not attracted to sweetpotatoes. Thus we suggest that sweetpotatoes are a nonpreferred host for SCB, and that management thresholds for control of adult populations must consider male/female ratio as well as moisture conditions.
Figure 1. Damage to sweetpotato root by southern corn rootworm.
Figure 2. Correlation of percentage of damaged sweetpotatoes with mean spotted cucumber beetle adults per 25 sweeps.
Figure 3. Scanning electron micrograph of a spotted cucumber beetle egg showing hexagonal pits that trap air and prevent drowning under wet conditions.
Figure 4. Total spotted cucumber beetles collected with a sweep net from two commercial sweetpotato fields in Mississippi during 2009.
Figure 5. Percentage of female spotted cucumber beetle adults collected from various hosts during 2009. N = total number of adults collected.
Insect management in Mississippi rice. J. Goreand D. Cook. Delta Research and Extension Center, Mississippi State University, Stoneville, MS.
The rice water weevil, Lissorhoptrus oryzophilus Kuschel, and the rice stink bug, Oebalus pugnax (F.), are the most important insect pests rice in Mississippi. Experiments were conducted in the Mississippi Delta to investigate these insects. Experimental seed treatments were evaluated to determine their efficacy against rice water weevil. Experiments were conducted at the Delta Research and Extension Center in Stoneville, MS and at Dulaney Farms near Clarkesdale, MS. The seed treatments evaluated included Dermacor and Cruiser at each location. All seed treatment and rate combinations significantly reduced rice water weevil larval populations on the roots of rice plants 3 to 4 weeks after flood. All rates of Dermacor resulted in significantly higher yields compared to non-treated plots and those that received a foliar application of Karate. Yield increases ranged from 10 to 35 bushels per acre.
Field cage experiments were conducted at the Delta Research and Extension Center in Stoneville, MS to determine the impact of rice stink bug on rice yields. In 2008, individual panicles were infested with 1 or 2 stink bugs. As a control, sleeve cages were placed over non-infested panicles. Rice stink bug injury was significantly higher where 2 stink bugs were caged per panicle compared to one stink bug per panicle and the non-infested panicle. Additionally, rice stink bug injury was greater where 1 stink bug was infested per panicle compared to the non-infested panicles. There were no significant differences in yields among the infested and non-infested panicles. However, there was a trend for decreasing yield with increasing stink bug infestation level. Based on regression analysis, there was a significant negative relationship between injury level and yield. This experiment was repeated in 2009. Panicles were infested during panicle emergence and continued each week until maturity.
Organophosphate resistance in the tarnished plant bug: population variation in major detoxification enzyme activity and gene expression. Yu Cheng Zhu. USDA-ARS Jamie Whitten Delta States Research Center, Stoneville, MS.
Transgenic Bt cotton has been adopted for more than 90% cotton area in the Mid-south areas to battle for a few lepidopteran insects, such as bollworm and tobacco budworm. As Bt cotton effectively controlled major lepidopetran insects, the tarnished plant bug and other sucking mouthpart insects have become serious problem because of reduced chemical applications and insecticide resistance development in these insects. Control of the tarnished plant bug in cotton in the mid-South relies heavily on pesticides, mainly organophosphates. Continuous and dominant use of chemical sprays has facilitated resistance development in the tarnished plant bug. Bioassay showed that esterase and glutathione S-transferase (GST) inhibitors significantly synergized toxicity of organophosphorous insecticide. Esterase and GST inhibitors also significantly suppressed esterase and GST enzyme activities in vitro. Field populations collected from Mississippi, Louisiana, and Arkansas were subjected to enzyme activity assay with α-naphthyl acetate (1-NA), β- naphthyl acetate (2-NA), ρ-nitrophenyl acetate (PNPA), 1-chloro-2,4,-dinitrobenzene, and acetylthiochroline. Results indicated that relatively big variations of esterase and GST activities and relatively low variation of acetylcholine esterase activity were detected among the different field populations. A total of 5 esterase cDNAs were cloned and sequenced by using cDNA library and RT-PCR. Real-time PCR examinations of esterase gene expressions revealed different esterase gene expression profiles in different field populations.
A Basic Design for Precision Agricultural Experiments. J. L. Willers, G. A. Milliken and J. N. Jenkins. USDA-ARS, Mississippi State, MS.
The objective of this presentation is to present a unique methodology for designing experiments that evaluate the effectiveness of a precision agricultural practice on a research farm field. We demonstrate an efficient method of combining the georeferenced treatment and georeferenced design structures necessary for carrying out and analyzing the site-specific experiment. Covariates (obtained by various remote-sensing systems) describe the field topography of the research field and or its crop attributes and apportion the field into various management zones. To each management zone in the field, only one of several rates (levels) from the set of site-specific treatments or treatment combinations is assigned. However, if the prescription is similar to the as-applied treatment implemented by the variable rate controller, then these management zones have geographical confounding among the prescribed and as-applied treatments. We describe a simple experimental design that resolves geographical confounding. Concepts are illustrated using a simplistic hypothetical field.
Student Competition
Phylogenetic relationships of the New World and Australian Schoenobiinae. E.L. Martinez and R.L. Brown. Dept. of Entomology and Plant Pathology, Mississippi State University.
The subfamily Schoenobiinae is a group of moths that inhabit marshy habitats. Most genera of Schoenobiinae are poorly known and only a few revisions that include descriptions of genitalia are available. Phylogenetic relationships of 13 genera of Schoenobiinae (Lepidoptera: Crambidae) are postulated based on traditional characters of genitalia and wing venation and new characters of the descaled whole body. The phylogenetic analysis yielded one most-parsimonious tree (length 287 steps, CI = 0.36, RI = 0.62) that resulted in a monophyletic clade of all genera of Schoenobiinae examined. The monophyly of the Schoenobiinae is supported by a Bremer support value of 5. The analysis confirms Lewvanich’s hypothesis that Scirpophaga, Donacaula, Schoenobius, Catagela, and Helonastes are closely related.
Exploring the Gopher Tortoise tick (Amblyomma tuberculatum) salivary gland transcriptome. P. Singh and S. Karim. Dept. of Biological Sciences, the University of Southern Mississippi.
Ticks are found in almost every region of the world and are second only to mosquitoes in their public health and veterinary importance. Ticks transmit the greatest variety of pathogens of humans, veterinary and wildlife species of any arthropod vector. The Gopher Tortoise tick (Amblyomma tuberculatum) is an aggressive ixodid tick which is in fact the largest known species of tick in United States and infest Gopherus polyphemus, one of the endangered and rare species of tortoise native to the coastal plains of the United States. The tick’s multifunctional salivary gland is vital to its biological success, and also plays a role in transmitting disease-causing agents to its hosts. Ticks deliver a repertoire of pharmacologically active compounds to the site of infestation that affects among other things, homeostasis and host immunity, thus facilitating the completion of a good quality meal for the tick. In this study, we have constructed normalized cDNA libraries from unfed and partially fed salivary glands from female adult ticks (Amblyomma tuberculatum). Initially small scale sequencing of selected clones revealed homologous transcripts of putative Histamine binding proteins, Serotonin and histamine binding proteins, salivary proteins, house-keeping genes and enzymes. Results of massive sequencing of unfed and partially fed cDNA libraries will be discussed in this presentation.
The lone star tick, Amblyomma americanum (Acari: Ixodidae) as a vector of tick-borne agents in Mississippi. A.H. Castellaw, J. Showers, J. Goddard, E.F. Chenney, A.S. Varela-Stokes. College of Veterinary Medicine, Dept. of Basic Sciences, Mississippi State University; Dept. of Entomology and Plant Pathology, Mississippi State University.
This study evaluated Amblyomma americanum (lone star tick) in Mississippi for the presence of Ehrlichia chaffeensis, causative agent of human monocytic ehrlichiosis, E. ewingii, causative agent of human and canine granulocytic ehrlichiosis, Borrelia lonestari, putative agent of “southern tick-associated rash illness” (STARI), Francisella tularensis, the agent of tularemia, and Rickettsia spp., particularly R. amblyommii, a suspected pathogen. We collected adult A. americanum from four regions of Mississippi: Northeast, Northwest, Southeast, and East. Of the ticks collected, 192 were dissected and DNA was extracted for nested PCR assays to detect the above bacteria. In all, 2.6% of ticks had evidence of Borrelia sp., 3.7% for E. chaffeensis, 6.3% for E. ewingii, and 43.5% for a Rickettsia species. As determined by sequencing, most Rickettsia spp. were R. amblyommii. In addition, 42 pools (total of 950) of larval A. americanum collected in Southwest Mississippi were tested for the presence of E. chaffeensis and Rickettsia species. Of the pools tested by PCR, 9 out of 42 (21.4%) were positive for R. amblyommii; none had evidence of E. chaffeensis, supporting the ability of LSTs to transovarially transmit R. amblyommii but not E. chaffeensis. This study demonstrates E. chaffeensis, E. ewingii, B. lonestari, and R. amblyommii in A. americanum by PCR for the first time in Mississippi. Understanding the prevalence and epidemiology of these agents in Mississippi should increase awareness of tick-borne disease in the medical community.
The role of small animals in the natural history of Rickettsia parkeri.G. Mararu, J. Goddard, A.S. Varela-Stokes. College of Veterinary Medicine, Dept. of Basic Sciences, Mississippi State University; Dept. of Entomology and Plant Pathology, Mississippi State University.
The Gulf Coast tick, Amblyomma maculatum, is the vector of the pathogenic bacterium, Rickettsia parkeri. Because the natural history of R. parkeri is poorly understood, it is prudent to determine what hosts this tick prefers and those on which it feeds successfully. This study considers these aspects using three potential species: anoles, quail, and rats. Whether they may serve as reservoir hosts for R. parkeri is also important, so quail and rats were used to determine their reservoir potential. In the host preference study, larvae or nymphs were given a choice of host. We recorded the number of ticks that engorged by feeding on hosts. For feeding success, we placed ticks directly onto each animal and allowed them to feed until engorged. We again recorded the number that engorged, the number that successfully molted, and the weights of engorged nymphs. To study R. parkeri infection, four quail and four rats were injected with the organism; one of each species received uninfected media as a negative control. We collected blood samples at various time points to test for antibodies to R. parkeri and rickettsial DNA; we also put larvae and later, nymphs on the animals. Because numbers were so low, no significant difference in terms of host preference between quail and rats could be seen. More engorged ticks were recovered from quail in the feeding success study, but those from rats weighed significantly more. We found no ticks that fed successfully on anoles, thus, anoles were not used in the infection study. Both quail and rats exposed to R. parkeri seroconverted by day post-infection 11, but none became rickettsemic. These results support a role for quail and rats in the natural history of R. parkeri, but the true extent of their role is still unclear.
Posters
A characterization of novel mitochondrial DNA markers for imported fire ant species. David C. Cross and Michael A. Caprio.
The hybrid imported fire ant Solenopsis invicta x richteri has expanded its range from the northern parts of MS and AL into eastern AR (unpublished data), TN, and northwest GA. Particularly in and near the hybrid zone, it is becoming increasingly difficult to identify which species or hybrid form one may have by using only morphological characters. Efforts at using biological control measures are especially dependent on knowing what species may predominate in a region. In an effort to develop more useful molecular tools directed towards simplifying identification, we have expanded the scope of use of S. invicta RFLP mitochondrial DNA markers described by Ross and Shoemaker (1997). We added an additional restriction enzyme, SspI, to four of theirs, BamHI, MspI, TaqαI, and HinfI and have found the five to be efficacious for delineating a total of six mtDNA haplotypes present in either the two parent species or the hybrid form. We tested populations in AR, AL, TN, MS, SC, GA, FL and CA. We found a single, unique S. richteri haplotype and five in S. invicta. The digestion patterns from any of the three restriction enzymes, SspI, BamHI or TaqαI could be used to distinguish between the two species’ haplotypes.
Stocking rate for laboratory reared tarnished plant bug, Lygus lineorlaris. Sarah Self, and John Schneider.
Currently ranked as the most economically damaging pest of cotton in the Mid-South, (Williams 2009), the tarnished plant bug (TPB), Lygus lineolaris, is of major concern to cotton production systems. Large quantities of quality TPB are required for conducting research on biological and chemical control tactics and technologies. Therefore, improving rearing techniques for TPB in an effort to increase efficiency and productivity, without sacrificing product quality, will become progressively more important over time. While rearing systems are generally successful, they are also conducted ad hoc. For example, rearing containers are stocked with oviposition packets containing an unknown number of eggs. One objective of our research project is to develop a stocking rate for TPB rearing systems. Our goal is to determine how many oviposition packets will produce the maximum number of adult females without sacrificing their overall health and viability. Our current colony is infected with a microsporidium in the genus Nosema. Unpublished data suggests that this infection does not affect the oviposition rate by TPB, but that it does appear to shorten their life-span (F. Musser, personal communication, October 23, 2009). Our stocking rates, established for infected individuals may, or may not be limited to other infected colonies based upon this information. Stocking rate experiments were conducted from April 2009 to September 2009. All experiments were conducted in rearing chambers in the MSU Rearing Center. TPB were reared on a 16:8 light/dark cycle, at 26.6°C, and at 55% relative humidity +/- 5%. All experiments were arranged in a replicated randomized complete block design. Four stocking rates, two, four, six and eight oviposition packets, were used as treatments. Due to variation in egg lay over time, rearing containers were only stocked with oviposition packets collected within the first 10 days of egg lay, at peak oviposition. Each treatment was applied to an individual rearing container, and was replicated three times in four separate containers, creating a total of 12 containers per experiment. Each experiment was repeated three times, for a total of 36 rearing containers. Total females produced were counted, and 30 randomly selected females were weighed in milligrams for each rearing container. Data was analyzed using SAS® 9.1.3 statistical software. Analysis of variance, simple linear regression, and least significant differences were calculated using a p-value of 0.05.
Total number of females produced increased numerically for each treatment level. However, treatment levels were not found to be statistically different with respect to total number of females produced. Female mass was used as a measure of product quality. As cohort sizes increased with treatment level, mass showed a linear decline across all treatment types. However, mass increased slightly from four to six oviposition packets, and then decreased from six to eight oviposition packets. Regression results showed a significant linear relationship between mass decline and treatment level (a < 0.0001), however, due to the variation in the six oviposition packet treatments, there was also a significant lack of fit in the regression (a = 0.0015). Least significant differences showed that only treatment levels of two oviposition packets were significantly different from the other treatment levels which were all linked. Mass decline showed a significant linear trend with treatment level. However, the variation between treatment levels suggests that more data will be needed to explain the relationship between cohort size and adult female weight in TPB. Additionally, total female production per rearing container has not yet appeared to reach a maximum level or carrying capacity. In light of this, further experiments are currently underway, testing new treatment levels of 10, 12 and 14 oviposition packets. It is hoped that this new data will help develop a new and reliable stocking rate for TPB rearing systems.Literature Cited:
Williams, M. R. 2009. Cotton Insect Losses 2008, in Beltwide Cotton Conference Proceedings, San Antonio, Texas, Jan. 5-8, pp 897-940.
Invasive and other exotic ants in Mississippi. Joe A. MacGown, Richard L. Brown, and JoVonn G. Hill.
As a result of ant collections made throughout Mississippi by the Mississippi Entomological Museum since 2002, various trapping programs of the USDA-APHIS and other government agencies, and samples submitted by pest control operators, 25 species of exotic ants have been documented to occur in the state. This represents one third of the introduced species reported from the entire Southeast. Many of the exotic species in Mississippi appear to have been introduced from neighboring states, rather than their native regions, which include Africa, Argentina, Brazil, Europe, Greater Antilles, the Indo-Pacific region, Japan, Mexico, Puerto Rico, and Southeast Asia. Introduced species may increase their range by natural dispersal, or with the inadvertent aid of humans. They may be transported in nursery stock, mulch, firewood, garbage, hay bales, yard debris or other material by trucks, boats, trains, and planes. Although not all exotic species have been shown to have obvious impacts, some are considered invasive and have negative effects on human health, the economy, agriculture, and natural ecosystems. Notable invasive species already established in the state include imported fire ants, Solenopsis invicta, S. richteri and their hybrid; Argentine ants, Linepithema humile; and pharaoh ants, Monomorium pharaonis. Other species recently reported or detected in Mississippi that have potential for becoming invasive include the ghost ant, Tapinoma melanocephalum; the dark rover ant, Brachymyrmex patagonicus; the crazy ant, Paratrechina longicornis; the Rasberry crazy ant, P. sp. nr. pubens; the snap-jaw ant, Odontomachus ruginodis; big headed ants, Pheidole moerens and P. obscurithorax; and the pavement ant, Tetramorium caespitum.