Research Article
A Novel Delivery Method for Ant (Hymenoptera: Formicidae) Toxicants [pdf]
Wiltz, B. A.1*, D. R. Suiter2, W. A. Gardner2, and C. W. Berisford3
1Southern Regional Research Center, USDA-ARS, 1100 Robert E. Lee Blvd, New Orleans, LA 70124
2Department of Entomology, University of Georgia, College of Agricultural and Environmental Sciences, Griffin Campus, 1109 Experiment Street, Griffin, GA 30223
3Department of Entomology, University of Georgia, Athens, GA 30602
*Corresponding author. Mailing address: Southern Regional Research Center, USDA-ARS, 1100 Robert E. Lee Blvd, New Orleans, LA 70124. email: beverly.wiltz@ars.usda.gov.
This article presents the results of research only. Mention of a commercial or proprietary product does not constitute endorsement or recommendation by the USDA.
Received: 4-I-2010 Accepted: 20-IV-2010
Abstract: Described here is a new delivery method for ant toxicants consisting of an inert carrier, an attractant, and a toxicant. Unlike baits, this system does not contain a food source, but uses ant to ant contact rather than trophallaxis as the mechanism for horizontal dispersal of the toxicant through the colony. We evaluated six potential attractants and found that only triolein increased treated filter paper removal by the red imported fire ant, Solenopsis invicta Buren (Hymenoptera: Formicidae). On corn cob grits, removal was optimized at a rate of 60 μl triolein/g grits. In laboratory assays, mortality of fire ants offered corn cob grits treated with a combination of fipronil and triolein was 90.5%, versus 46.5% when grits were treated with the same rate of fipronil without triolein. In both lab and field trials, the removal of grits treated with a combination of fipronil and triolein was greater than removal of controls but less than removal of grits treated with triolein alone.
Key words: fire ant, horizontal toxicity, attractant, triolein
Introduction
Baits take advantage of social behavior to control ant colonies efficiently by using relatively little toxicant. An effective bait contains an active ingredient that has delayed toxicity, is non-repellent, and is effective over a range of concentrations (Stringer et al. 1964, Williams 1983). Most commercially available fire ant baits use a vegetable oil, such as soybean oil, that acts as a phagostimulant and as a solvent for the active ingredient. The toxicant and attractant are absorbed onto an inert carrier. Liquid and gel ant baits contain sugar as a food source, along with toxicants that are more water soluble.
Despite the widespread use of baits for ant control, there remains a need for attractive formulations because currently available baits are not consistently accepted by some species, such as the black carpenter ant Camponotus pennsylvanicus (DeGeer) (Tripp et al. 2000). Although baiting has been practiced for control of the red imported fire ant, Solenopsis invicta Buren, for over 40 years (Williams et al. 2001), problems still exist because of seasonal changes in bait preferences (Stein et al. 1990). Additionally, baits generally lack species specificity because of their food-based attractants.
For both the red imported fire ant and the Argentine ant, Linepithema humile (Mayr), there can be a high level of horizontal mortality due to contact between untreated ants and insecticide-treated corpses (Soeprono and Rust 2004, Choe and Rust 2008, Wiltz et al. 2009, 2010). We propose that with the addition of an attractant, a bait-like formulation can be effective without a food source, using physical contact as the mechanism of horizontal transfer. While the concept is applicable to the control of other social insects, the red imported fire ant was selected for this study because it is a major economic pest, its chemical ecology has been extensively studied, and all life stages are readily available.
Pheromones have been reported to enhance recruitment to or consumption of baits by ants. Vander Meer (1996) found that invictolide, a component of the fire ant queen-recognition pheromone, decreased the discovery time and increased the number of bait particles discovered. The addition of Argentine ant trail pheromone to sucrose solution enhanced consumption, demonstrating a potential method of improving liquid bait consumption and specificity (Greenberg and Klotz 2000). Other types of chemicals that can potentially be used to entice ants to remove treated particles include those that elicit feeding, necrophoric, or brood-tending behaviors. Fatty acids have been shown to elicit both feeding and necrophoric responses. Bomar and Lockwood (1994) found that linoleic and linolenic acids attracted multiple grasshopper species, a seed bug, and five ant species, and that the addition of linoleic acid increased the efficacy of baits against grasshoppers. Oleic acid was reported to induce necrophoric behavior in imported fire ants and the harvester ant Pogonomyrmex badius (Wilson et al. 1958, Gordon 1983). While these fatty acids also occur in living insects, necrophoresis can be inhibited by the presence of additional chemicals. For example, the pygidial gland products dolichodial and iridomyrmecin are present in live Argentine ants but disappear from the cuticular surface within 1 h of death (Choe et al. 2009). Removal of dead nestmates begins after the disappearance of these chemicals. Interspecific aggression is inhibited by similar chemicals produced by other species (Tomalski et al. 1987, Völkl et al. 1994). Bigley and Vinson (1975) reported that diolein and triolein were responsible for brood-tending behavior by S. invicta workers. It has since been argued that brood recognition likely results from a combination of physical and chemical cues and that these two chemicals are more likely to elicit a food response than a brood-recognition response (Vander Meer 1983, Morel and Vander Meer 1988). For the purposes of this study, either type of response would be expected to have the desired effect of increasing the number of treated particles carried into the nest.
Materials and Methods
This study consisted of four parts: screening potential attractants on filter paper, determining the optimum rate on corn cob grits, evaluating mortality of lab colonies due to handling grits treated with attractant and toxicant, and evaluating removal of treated particles by field colonies.Test Insects. Red imported fire ant colonies were collected in Spalding County, GA. Ants were separated from soil by connecting two 19 × 13 × 10 cm Fluon™ (Northern Products, Inc., Woonsocket, RI)-lined plastic boxes (Pioneer Plastics, Dixon, KY) with paper bridges (10 × 50 cm strips of cardstock, with the ends taped to the bottom of each box). Soil containing fire ants was placed in one box and nest cells, food, and water in the other. Nest cells were constructed from 100 × 25 mm Petri dishes containing a 1.0-cm thick layer of hardened dental plaster (Castone; Dentsply International Inc., York, PA) to retain moisture. Dish sides and lids were painted black and three 4-mm holes were drilled into the sides to allow ants to enter. As soil dried, workers moved the entire colony to the nest cells. Nest cells containing ants were transferred to clean 31 × 23 × 10 cm Fluon-lined plastic boxes and colonies maintained at room temperature (23–26 °C) on a diet of water, 25% sugar water, and frozen house crickets (Acheta domesticus).
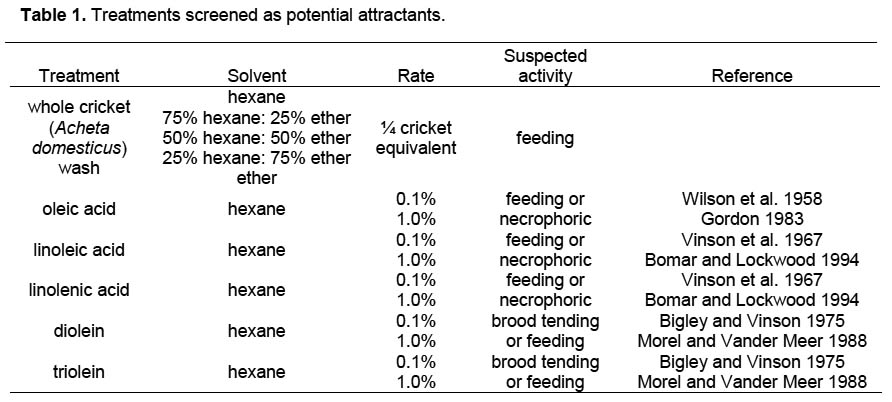
Attractant Screening. Initial screening was conducted using small lab colonies. A nest cell containing approximately 5 g workers with brood was placed at one end of a 19 x 13 x 10 cm Fluon-lined plastic box. Two-choice tests were conducted by placing treated filter paper circles (Whatman #3, punched with a 1/8” hole punch) on scoops (approximately 30 x 20 mm bottom with 20 mm handle) cut from plastic weigh boats and placing the scoops at the opposite end of the box from the nest cell. This provided a flat surface from which to evaluate the removal of filter paper circles and allowed choices to be easily placed into the box simultaneously. Treatments were selected for their potential to elicit responses from S. invicta that would increase particle handling. Objects treated with compounds eliciting predatory or brood-tending responses were expected to be carried into the nest, while those eliciting necrophoric behavior were expected to be carried away from the nest. Six potential attractants were evaluated (Table 1). Washes of whole crickets, Acheta domesticus, were selected because we suspected that they would elicit a feeding response. Because the cricket cuticle could contain multiple components that signal the presence of prey, crickets were washed in five solvents: hexane, ether, and three combinations of the two. Further separation and analysis of active fractions would be conducted if any fraction enhanced handling of treated filter papers in the initial screening. The remaining compounds were selected based on literature reports that they elicit feeding, necrophoric, or brood-tending responses. They were each evaluated at two rates, resulting in a total of 15 treatments. Unless otherwise indicated, all chemicals were dissolved in hexane. Each replicate received ten filter paper circles treated with 5 μl of one of the test materials, paired with a control of 10 filter paper circles treated with the same solvent used for the treated disks. Before applying the chemicals, either treated or control circles received a pencil mark so the choices could be distinguished from each other within the box. At 10-min intervals for 1 h, the number of filter papers removed from the plastic scoop and the number brought into the nest were recorded. Tests were replicated nine times for each treatment.
Rate determination. Triolein was selected for further testing based on the results of filter paper assays. A no-choice test was conducted to determine the rate of triolein that elicited the greatest response when applied to corn cob grits. Lab colonies were provided 10 grits treated with one of eight rates of triolein: 0.01, 0.05, 0.1, 0.15, 0.2, 0.25, 0.3, or 1.0 μl. Controls were treated with 10 μl hexane. For this test and all that follow, corn cob grits were cleaned prior to use by triple washing with hexane and air drying for at least 1 d.
Triolein was dissolved in hexane for a total volume of 10 μl per grit and applied to individual 10–14 mesh corn cob grits (The Andersons, Maumee, OH) by using a Hamilton syringe. This grit size was selected because it is the preferred food particle size for red imported fire ants (Hooper-Bùi et al. 2002). Grits were air dried for 30 min to 1 h after treatment, then 10 grits were placed on a plastic scoop in a 19 × 13 × 10 cm Fluon-lined plastic box containing a nest cell with approximately 5 g fire ant workers + brood. The time until each grit was removed and the time until it was brought into the nest within 30 min were recorded. Six replicates were conducted for each rate.
Laboratory evaluation with toxicant. Based on the results of the above test, a rate of 0.15 μl triolein per grit was selected for evaluation on grits with toxicant. Fipronil was used as the active ingredient for this assay because it is non-repellent, slow-acting, and has high contact and horizontal toxicity (Wiltz et al. 2009, 2010). Grits were treated with 0.1, 0.2, 0.3, 0.4, 0.5, or 0.6% fipronil (fipronil weight/grit weight) + 0.15 μl triolein per grit, 0.15 μl triolein per grit, 0.06% fipronil, or hexane (control). Corn cob grits for all fipronil treatments were treated by mixing the appropriate volume of Termidor SC (9.1% fipronil, BASF, Research Triangle Park, NC) in water to make a total volume of 2 ml, then adding to 1 g grits in a glass Petri dish. Grits were dried 24 h under a fume hood, with occasional stirring to facilitate drying. Triolein was applied to individual grits as described above, with the triolein being applied to dry fipronil-treated grits in treatments containing both chemicals. Controls were treated with 20 μl hexane. Assays were conducted in 19 × 13 × 10 cm Fluon-lined plastic boxes, each containing a nest cell with 3 g fire ant workers and a water-soaked cotton ball on a 45-mm plastic lid. The water source was placed on top of the nest cell and a weigh boat scoop with 50 treated grits was placed on the opposite side of the box. Boxes were covered with lids containing two 6-mm holes and maintained at 24 °C with a photoperiod of 12: 12 (L: D) for 3 d when the numbers of dead and alive ants and the locations of grits (not moved, on box floor, or in nest cell) were recorded.
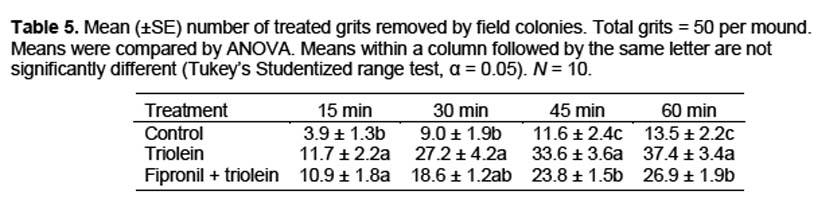
Removal by field colonies. Removal of particles by field colonies was evaluated by using corn cob grits treated with either fipronil + triolein, triolein alone, or hexane (control). Fipronil was applied to grits at a rate of 0.06% and grits were dried under a fume hood for 24 h before treatment with triolein. Triolein was applied at a rate of 60 μl/g grits, the equivalent of the 0.15-μl rate used in the previous tests. In both the fipronil and no fipronil treatments, triolein was dissolved in hexane and then sprayed onto the grits in a glass dish by using an aromatherapy atomizer (Aura Cacia, Urbana, IA). Grits were sprayed five times, swirled to mix, and repeated until all of the triolein solution was used, to provide a uniform coating to all grits without removing the fipronil. Field trials were conducted 1 to 2 h after triolein treatment. Treatments were randomly assigned to mound numbers in advance. Each mound encountered that had actively foraging ants was then assigned a sequential number. To quantify grit removal, 50 treated grits were placed on a 50-mm diameter plastic disk adjacent to a fire ant foraging trail. The number of grits remaining on the disk was recorded at 15-min intervals for 1 h. All tests were conducted in the morning or evening when foraging ants could be easily located. Air temperatures ranged from 21–24° C. Ten colonies per treatment were evaluated.
Statistical analysis. Results of filter paper choice tests were analyzed by using two-tailed paired t-tests. For the fipronil-treated grit assay, mortality and particle location data for all treatments were compared by using one-way analysis of variance (ANOVA) and means were separated with Tukey’s studentized range test (SAS Institute 1985). Mortality was analyzed by using probit analysis (PROC PROBIT; SAS Institute 1985) to determine an LD90 of fipronil on corn cob grits with triolein added. For field trials, the cumulative numbers of grits removed in each treatment were compared at each time period by using ANOVA and means were separated with Tukey’s studentized range test.
Results
Attractant Screening. Control filter papers were removed in the choice tests containing both rates of linoleic acid, linolenic acid, diolein, and triolein, but few were removed in any of the cricket wash or oleic acid treatments. In treatments where control filter papers were moved, few were ever brought into the nest. Only one treatment, 1% triolein, significantly increased filter paper removal relative to the paired controls (Table 2). At each time period, both the cumulative number of filter paper circles carried into the nest and the total cumulative number removed (inside nest + outside nest) were greater for the 1% triolein treatment than the controls. Both rates of linoleic acid, 0.1% linolenic acid, and 1.0% diolein each had fewer cumulative filter papers removed (total inside nest + outside nest) than controls for at least one time period.
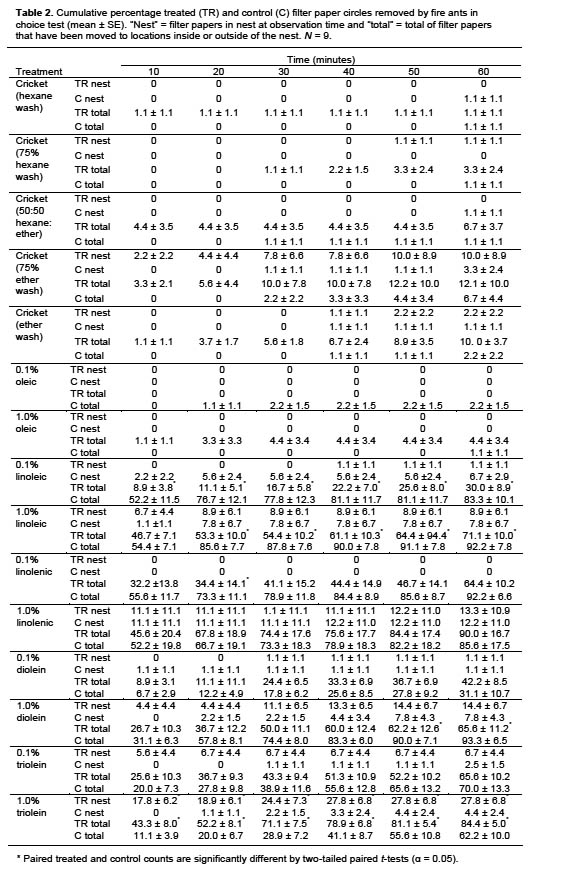
Rate determination. Two of the triolein rates tested had all 10 treated grits brought into the nest after 30 min: 0.10 and 0.15 μl triolein per grit (Table 3). Of these rates, the mean time for all grits to be removed and brought into the nest was slightly lower with 0.15 μl than with 0.1 μl triolein. Therefore, the 0.15 μl rate was selected for subsequent tests.
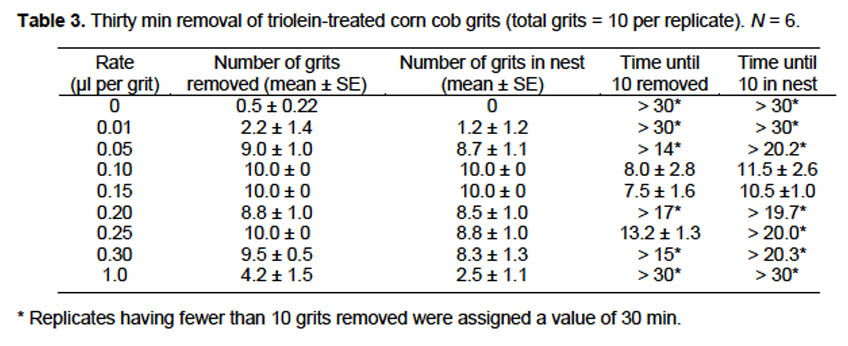
Laboratory evaluation with toxicant. When 3 g fire ant workers were provided corn cob grits treated with fipronil and 0.15 μl triolein per grit, the LD90 (95% CI) was 0.059% (0.053–0.066%) fipronil (per weight of corn cob grits). More important than the actual rate, we found that versus grits treated with fipronil alone, mortality doubled when grits were treated with fipronil plus triolein (Table 4). Fire ant mortality increased to 90.5% when triolein was added to grits with 0.06% fipronil, versus 46.5% mortality with the same rate of fipronil alone. Mortality in the 0.06% fipronil-only treatment did not differ from the 0.01, 0.02, or 0.03% fipronil with triolein treatments. Ants were observed bringing treated corn cob grits into nest cells within minutes of placement in the boxes. However, few grits in any of the treatments remained inside the nest after 3 d.
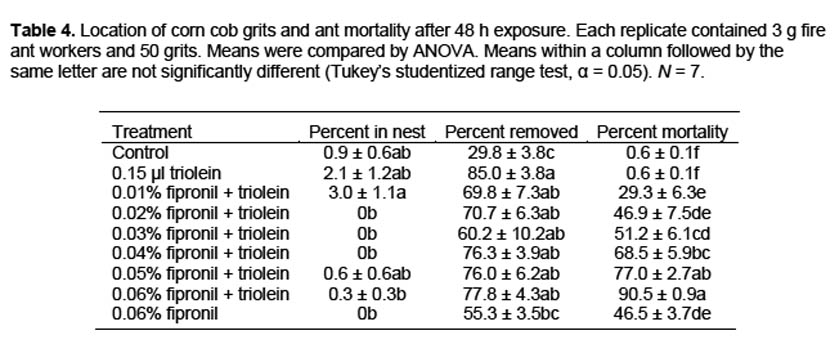
Removal by field colonies. At all time periods, removal of triolein-treated grits was greater than removal of control grits (Table 5). Removal of grits treated with fipronil and triolein was greater than removal of controls at all time periods except 30 min, but was less than removal of triolein treated grits at 45 and 60 min.
Discussion
We have demonstrated that the addition of triolein to corn cob grits 1) increased the number of particles brought into the laboratory and field colonies of S. invicta and 2) increased mortality relative to particles treated with an active ingredient alone. This technique has potential to provide a more species specific ant control method and to alleviate the problem of variable food preferences that makes baiting of some species difficult.
When treated filter papers were removed, they were usually placed outside the nest. The 1% triolein treatment had only 27% of filter papers in the nest, but this was a significant improvement over controls and high relative to other treatments. While low percentages inside the nest would seem to suggest a necrophoric response, this result could be because the ants experienced difficulty handling the filter paper disks. This possibility is supported by higher in-nest percentages for corn cob grits than for filter papers. Ants removed control disks in linoleic, linolenic, diolein, and triolein choice tests, but few control disks were removed in oleic and cricket wash treatments. When untreated filter paper disks were moved, few were carried into the nest. Removal of control disks corresponded with a general trend towards ants leaving the nest more in some treatments than in others (unquantified observation) and can possibly be attributed to the presence of volatiles from the paired treated disks.
In 48-h mortality tests, 85% of triolein-treated corn cob grits had been removed. However, only 2% were found inside the nest. In field trials using 1, 5, or 25 g of treated grits, we did not observe any colony mortality 10 d after treatment (unpublished data). At this time, many of the grits remained, or had been returned, outside the nest. These results suggest two additional areas that must be investigated further. First is the stability of triolein. While particles treated with triolein were observed being removed by ants in both laboratory and field trials, few remained in the nest after 3 d in the laboratory assay. Bigley and Vinson (1975) found that while 100% of triolein treated disks were inside fire ant colonies after 30 min, only 75% remained after 4 h. Another factor that might negatively impact field efficacy is the active ingredient formulation. Removal by laboratory and field colonies was less for triolein-treated grits with fipronil than without fipronil. While this might be due in part to sublethal effects of fipronil, there are likely other factors involved because removal rate of fipronil-treated grits was at least as high as the removal of controls. Fipronil residues are non-repellent to ants (Wiltz et al. 2009, 2010). Therefore, we suspect that the reduction in grit handling could be due to the accumulation of Termidor SC inert ingredients on the surface of corn cob grits. However, we were not able to test this hypothesis because technical grade fipronil was not available for evaluation.
Rates of removal by field colonies were lower than removal of the same treatments under laboratory conditions. Ants are known to have different reactions to the same chemical, depending on current nest activities (Gordon 1983). Temperature affects both foraging and necrophoric behavior (Vogt et al. 2003, Challet et al. 2005). Other factors that might contribute to different removal rates by laboratory and field colonies include group size, proximity of the objects to the nest, and humidity-induced changes in the surface texture of treated particles.
Areas for future studies include evaluating different toxicants, identifying materials that are attractive to other species, and evaluating species specificity. Numerous possibilities exist for attractants for either red imported fire ants or other species. Obin and Vander Meer (1994) demonstrated that S. invicta workers preferentially entered, searched, and recruited nestmates to vials containing either an alate corpse or alate residue. Alonso and Vander Meer (1997) later determined that the alate mandibular glands are the source of these excitant pheromones. Vander Meer et al. (1980) discovered that in S. invicta, the attractants and queen recognition pheromones are stored in the poison sac and dispensed by the sting apparatus. The pheromone consists of minor non-alkaloid components of the poison sac contents. Components of the S. invicta trail pheromone include Z,E-α-farnesene, E-E-α-farnesene, Z-E- homofarnesene, Z-Z-homofarnesene, and Z-Z-Z-allofarnesene (Vander Meer et al. 1981, Williams et al. 1981). Vander Meer (1983) found that similar trail-following behavior was elicited by Z,E-α-farnesene alone as by the Dufour’s gland extract. While trail pheromones have been studied more extensively in S. invicta than in other species, they are known for several other ant species, including Monomorium pharaonis (L.) (Ritter et al. 1977), Lasius fuliginosus (Latrielle) (Huwyler et al. 1975), Atta texana (Buckley) (Tumlinson et al. 1971), and Atta sexdens rubropilosa Forel (Cross et al. 1979). More work must be done to evaluate the species specificity of response to triolein. In addition positive results with S. invicta in this study and the work of Bigley and Vinson (1975), triolein elicited a food response in seed-dispersing ants, with treated particles removed at a similar rate to intact eliasomes (Brew et al. 1989). However, we found that triolein did not enhance filter paper handling by L. humile at 0.1 or 1.0% (unpublished data). Despite the lack of field efficacy with the current formulation, positive results obtained in the lab studies and particle removal by field colonies suggest that this technique can be effective if further developed.
References
Alonso, L. E., and R. K. Vander Meer. 1997. Source of alate excitant pheromones in the red imported fire ant Solenopsis invicta (Hymenoptera: Formicidae). J. Insect Behav. 10: 541-555.
Bigley, W. S., and S. B. Vinson. 1975. Characterization of a brood pheromone isolated from sexual brood of the imported fire ant, Solenopsis invicta. Ann. Entomol. Soc. Am. 68: 301-304.
Bomar, C. R., and J. A. Lockwood. 1994. Olfactory basis of cannibalism in grasshoppers (Orthoptera: Acrididae): II. Field assessment of attractants. J. Chem. Ecol. 20: 2261-2272.
Brew, C. R., D. J. O'Dowd, and I. D. Rae. 1989. Seed dispersal by ants: behaviour-releasing compounds in elaiosomes. Oecologia 80: 490-497.
Challet, M., C. Jost, A. Grimal, J. Lluc, and G. Theraulaz. 2005. How temperature influences displacements and corpse aggregation behaviors in the ant Messor sancta. Insectes Sociaux 52: 309-315.
Choe, D-H. and M. K. Rust. 2008. Horizontal transfer of insecticides in laboratory colonies of the Argentine ant (Hymenoptera: Formicidae). J. Econ. Entomol. 101:1397-1405.
Choe, D-H., J. G. Millar, and M. K. Rust. 2009. Chemical signals associated with life inhibit necrophoresis in Argentine ants. PNAS 106: 8251-8255.
Cross, J. H., R. C. Byler, U. Ravid, R. M. Silverstein, S. W. Robinson, P. M. Baker, J. Sabino de Oliveira, A. R. Jutsum, and M. J. Cherrett. 1979. The major component of the trail pheromone of the leaf-cutting ant, Atta sexdens rubropilosa Forel. 3-ethyl-2,5-dimethylpyrazine. J. Chem. Ecol. 5: 187-203.
Gordon, D. M. 1983. Dependence of necrophoric response to oleic acid on social context in the ant, Pogonomyrmex badius. J. Chem. Ecol. 9: 105-111.
Greenberg, L., and J. H. Klotz. 2000. Argentine ant (Hymenoptera: Formicidae) trail pheromone enhances consumption of liquid sucrose solution. J. Econ. Entomol. 93: 119-122.
Hooper-Bùi, L. M., A. G. Appel, and M. K. Rust. 2002. Preference of food particle size among several urban ant species. J. Econ. Entomol. 95: 1222-1228.
Huwyler, S., K. Grob, and M. Viscontini. 1975. The trail pheromone of the ant, Lasius fuliginosus: identification of six components. J. Insect Physiol. 21: 299-304.
Morel, L., and R. K. Vander Meer. 1988. Do ant brood pheromones exist? Ann. Entomol. Soc. Am. 81: 705-710.
Obin, M. S., and R. K. Vander Meer. 1994. Alate semiochemicals release worker behavior during fire ant nuptial flights. J. Entomol. Sci. 29: 143-151.
Ritter, F. J., I. E. M. Brüggemann-Rotgans, P. E. J. Verwiel, C. J. Persoons, and E. Talman. 1977. Trail pheromone of the Pharaoh's ant, Monomorium pharaonis: isolation and identification of faranal, a terpenoid related to juvenile hormone II. Tetrahedron Letters 18: 2617-18.
SAS Institute. 1985. SAS user’s guide: statistics, 5th ed. SAS Institute, Cary, NC.
Soeprono, A. M. and M. K. Rust. 2004. Effect of horizontal transfer of barrier insecticides to control Argentine ants (Hymenoptera: Formicidae). J. Econ. Entomol. 97: 1675-1681.
Stein, M. B., H. G. Thorvilson, and J. W. Johnson. 1990. Seasonal changes in bait preference by red imported fire ant, Solenopsis invicta (Hymenoptera: Formicidae). Fla. Entomol. 73: 117-123.
Stringer, C. E., Jr., C. S. Lofgren, and F. J. Bartlett. 1964. Imported fire ant bait studies: Evaluation of toxicants. J. Econ. Entomol. 57: 941-945.
Tomalski, M. D., M. S. Blum, T. H. Jones, H. M. Fales, D. F. Howard, and L. Passera. 1987. Chemistry and functions of exocrine secretions of the ants Tapinoma melanocephalum and T. erraticum. J. Chem. Ecol. 13: 253-263.
Tripp, J. M., D. R. Suiter, G. W. Bennett, J. H. Klotz, and B. L. Reid. 2000. Evaluation of control measures for black carpenter ant (Hymenoptera: Formicidae). J. Econ. Entomol. 93: 1493-1497.
Tumlinson, J. H., R. M. Silverstein, J. C. Moser, R. G. Brownlee, and J. M. Ruth. 1971. Identification of the trail pheromone of a leaf-cutting ant, Atta texana. Nature 234: 348-349.
Vander Meer, R. K. 1983. Semiochemicals and the red imported fire ant (Solenopsis invicta Buren) (Hymenoptera: Formicidae). Fla. Entomol. 66: 139-161.
Vander Meer, R. K. 1996. Pheromone enhanced baits for pest ant control: Current status and future prospects, pp. 531-539. In K. B. Wildey [ed.], Proceedings of the 2nd International Conference on Insect Pests in the Urban Environment. BPCC Wheatons Ltd., Exeter, UK.
Vander Meer, R. K., B. M. Glancey, C. S. Lofgren, A. Glover, J. H. Tumlinson, and J. Rocca. 1980. The poison sac of red imported fire ant queens: Source of a pheromone attractant. Ann. Entomol. Soc. Am. 73: 609-612.
Vander Meer, R. K., D. F. Williams, and C. S. Lofgren. 1981. Hydrocarbon components of the trail pheromone of the red imported fire ant, Solenopsis invicta. Tetrahedron Letters 1651-1654.
Vinson, S. B., J. L. Thompson, and H. B. Green. 1967. Phagostimulants for the imported fire ant, Solenopsis saevissima var. richteri. J. Insect Physiol. 13: 1729-1736.
Vogt, J. T., W. A. Smith, R. A. Grantham, and R. E. Wright. 2003. Effects of temperature and season on foraging activity of red imported fire ants (Hymenoptera: Formicidae) in Oklahoma. Environ. Entomol. 32: 447-451.
Völkl, W., G. Hübner, and K. Dettner. 1994. Interactions between Alloxysta brevis (Hymenoptera, Cynipoidea, Alloxystidae) and honeydew-collecting ants: How an aphid hyperparasitoid overcomes ant aggression by chemical defense. J. Chem. Ecol. 20: 2901-2915.
Williams, D. F. 1983. The development of toxic baits for the control of the imported fire ant. Fla. Entomol. 66: 162-172.
Williams, H. J., M. R. Strand, and S. B. Vinson. 1981. Synthesis and purification of the allofarnesenes. Tetrahedron 37: 2763-2767.Williams, D. F., H. L. Collins, and D. H. Oi. 2001. The red imported fire ant (Hymenoptera: Formicidae): an historical perspective of treatment programs and the development of chemical baits for control. Am. Entomol. 47: 146-159.
Wilson, E. O., N. I. Durlach and L. M. Roth. 1958. Chemical releasers of necrophoric behavior in ants. Psyche 65: 108-114.
Wiltz, B. A., D. R. Suiter, and W. A. Gardner. 2009. Activity of bifenthrin, chlorfenapyr, fipronil, and thiamethoxam against Argentine ants (Hymenoptera: Formicidae). J. Econ. Entomol. 102: 2279-2288.
Wiltz, B. A., D. R. Suiter, and W. A. Gardner. 2010. Activity of bifenthrin, chlorfenapyr, fipronil, and thiamethoxam against red imported fire ants (Hymenoptera: Formicidae). J. Econ. Entomol. 103 (in press).
