Research Article
Utilizing Diapause in a Sugarcane Borer (Lepidoptera: Crambidae) Laboratory Colony as a Cost Saving Measure [pdf]
White, W. H.
USDA, ARS Sugarcane Research Laboratory, 5883 USDA Road, Houma, LA 70360. Phone: (985) 853-3176 Fax: (985) 868-8369 william.white@ars.usda.gov
Received: 7-IV-2008; Accepted: 16-VI-2008
Abstract: The ability to rear insects in the laboratory broadens the scope of research opportunities available to the scientist. Our laboratory routinely rears the sugarcane borer, Diatraea saccharalis (F.), for research in host plant resistance and biological control of this important sugarcane pest. Unfortunately, insect rearing is a costly process. One option to reduce cost is to hold a founder population in diapause during times when insects are not needed. These insects can then be brought out of diapause and allowed to reconstitute the colony. A procedure was developed to put larvae of the sugarcane borer into diapuase, hold these larvae for five months in cold storage, and then terminate diapause and allow the insects to complete development. The larvae were put into diapause by holding them at 18°C with a photoperiod of 12:12 (L:D) h. The diapausing larvae were then held for five months at 10°C and a photoperiod of 12:12 (L:D) h. In 2007 our laboratory diapaused 1024 sugarcane borer larvae, terminated our routine rearing, and held the founder colony until January of 2008. Seventy-three percent of the founder colony that diapaused in 2007 survived and produced healthy moths in 2008. We were not able to detect any adverse affects (i.e. malformed pupae) in the diapaused insects and we resumed normal rearing operations within 30 days of initiating diapause termination. This procedure saved our laboratory approximately $1600.00 in rearing supplies, but more importantly it allowed us to redirect our insectary manager to other critical tasks during that period of time that insects were not needed.
Keywords: insect rearing, methodology, stemborers, parasitoid diapause
Introduction
The option of rearing insects in the laboratory broadens the scope of research opportunities available to the scientist. For example: research can be conducted year round; insects rare in nature can be made readily available; insect development can be synchronized so prescribed life stages can be obtained when needed; and highly selected populations can be created. Some insect species adapt readily to laboratory situations and are reared in large numbers. Singh (1994) reported that a variety of insects are produced in millions and used in various pest control programs. Undoubtedly, a variety of insects are difficult to rear and hence are produced in limited numbers. For example, in our laboratory, procedures to rear Diatraea evanescens Dyer (Lepidoptera: Crambidae) are more complicated and time consuming than those used to rear the sugarcane borer, D. saccharalis (F.) (Lepidoptera: Crambidae), and the numbers we produce of each reflect those differences. Regardless of the insect species and the ease (or difficulty) of rearing, insect rearing is expensive (i.e. labor, equipment, supplies, facilities) to conduct. To lower cost, much research has been conducted to increase rearing efficiency such as development of better diets and automated rearing equipment.
An obvious cost saving option available to the researcher is to curtail or cease rearing operations during periods of time when there is little or no need for reared insects. However, the consequences of adopting this strategy can be the outright loss of a selected colony, difficulty in collecting founder individuals to create a new colony, and an unacceptable delay in obtaining the required numbers of insects for planned research.
Another option for the researcher is to hold a founder colony in a diapause state during the period of low insect demand. Davis (1983) reported a simple and effective technique for storing southwestern corn borer, Diatraea grandiosella (Dyer) (Lepidoptera: Crambidae), in diapause. The sugarcane borer has also been shown to undergo facultative diapause (Katiyar and Long 1961). However, no one has exploited this aspect of the sugarcane borer’s biology as a means of storing larvae for an extended period of time. A procedure is reported to induce sugarcane borer larvae into diapause and hold them in the laboratory for up to five months before terminating diapause and ultimately the resumption of rearing.
Materials and Methods
Sugarcane borer have been reared continuously at the Sugarcane Research Laboratory since 1983. Our rearing procedures follow those developed for the southwestern corn borer as reported by Davis (1976) and as later modified by Davis et al. (1990). In our colony, neonate larvae are placed on 7 ml of sugarcane borer rearing media (Southland Products, Lake Village, AR) dispensed in 32 cell rearing trays (Mayfield Papers, San Angelo, TX). Each tray is covered and heat sealed with a plastic sheeting (Oliver Products, Grand Rapids, MI). Non-diapausing larvae require approximately 30 days to pupate at 26°C, 50% RH, and a photoperiod of 14:10 (L:D) h.
In 2006, eighteen, 32-cell trays were set up as four cohorts as a preliminary evaluation on the following dates: 25 July (six trays), 25 August (six trays), 28 August (three trays) and 1 September (three trays). Larvae were held for 10 days under routine insectary conditions and at this time all larvae should be third instar (Hensley 1960). We then followed the procedures of Fuchs et al. (1979) to induce diapause in the larvae. These authors reported the critical day-length for diapause induction in the sugarcane borer to be between 12 and 13 h of light when held at 21°C. Diapause was induced at 18°C, but after 60 days we stored diapausing larvae at 9°C and a photoperiod of 12:12 (L:D) h as we considered it necessary to go beyond the critical temperature for diapause induction since larvae would be held for up to five months. Trays were stored within the growth chamber in plastic boxes containing a dampened cotton ball. As the growth chamber did not have humidity controls, humidity within the chamber fluctuated with ambient conditions.
In this initial evaluation we did not attempt to determine the maximum time that larvae could be held, but simply terminated diapause when the laboratory returned to full staff (17 January 2007). This was accomplished by removing all larvae from the growth chamber and placing them in the insectary at 26°C and a photoperiod of 14:10 (L:D) h. From each cohort we randomly identified 20 cells and recorded the following data from these cells: sex of pupae, weight of pupae, and days to pupation. Cells not producing pupae were also recorded. Cohort adults were paired and allowed to mate and the resulting egg laying and percent egg hatch were monitored.
In 2007, based upon the positive results from 2006, we decided to put more larvae into diapause and terminate our rearing operation for the remainder of the year. Beginning on 3 September and continuing until 13 September (7 cohorts) a total of 1024 neonate larvae were placed on diet. These larvae were placed 1 per cell in 32 rearing trays. Larvae were held three days in the insectary at 26°C and a photoperiod of 14:10 (L:D) h and then transferred to a growth chamber set at 18°C and a photoperiod of 12:12 (L:D) h where they were held for 22 days. Finally, the larvae were transferred to a second growth chamber set at 10°C and a photoperiod of 12:12 (L:D) h and held for an additional 91 days (7 January 2008) when diapause was terminated. For each 32-cell tray, records were kept on the fate of each larva. These data included: mortality, days to pupation, sex of pupae, and weight of pupae. Random samples of egg masses were also taken noting number of eggs per egg mass and percent egg hatch.
Results and Discussion
We were successful in inducing and terminating diapause in larvae from our sugarcane borer colony. All four cohorts from 2006 had greater than 90% pupation following diapause termination. The cohorts had the following pupation success rates (%) (n = number larvae, d = larvae died, l = remained larvae, p = number pupae): cohort 1 = 90% (n = 192; d = 3; l = 4; p = 185); cohort 2 = 91% (n = 192; d = 7; l = 5;p = 180); cohort 3 = 93% (n = 96; d = 2; l = 5; p = 89); and cohort 4 = 92% (n = 96; d = 6; l = 2; p = 88). The ratio of M:F pupae among the four cohorts was approximately 1:1. There was little difference in male and female pupal weight among the four cohorts as evidenced by the small standard errors associated with means; however, female pupae were ≈ 27% heavier than the male pupae (Figure 1). During this same period of time, female pupae from the existing colony averaged 170 mg (≈ 20% more than diapaused females), while males averaged 80 mg (≈ 25% less than diapaused males). We are not certain why these differences existed; however, age of pupae at weighing may have produced these differences. Pupae lose weight as they mature and we had not standardized time of weighing.
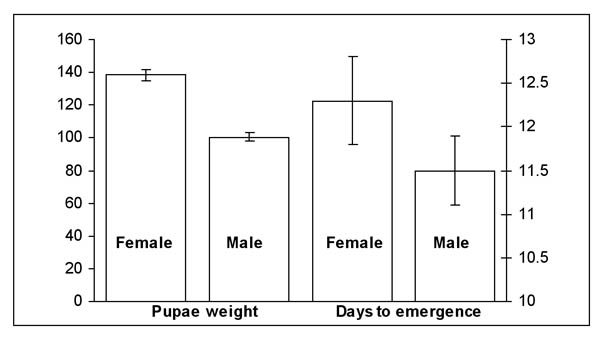
Figure 1. Mean weight (± sem) and days for moth emergence (± sem) of female and male pupae from four larval cohorts placed in diapause in 2006 and removed in 2007.
In the 2006 evaluation, there was also little difference in the number of days to pupation following termination of diapause among the four cohorts, but on average female pupae required one day longer to pupate than male pupae (Figure 1). Because male moths eclose sooner than female moths, we had difficulty in pairing moths to evaluate egg viability. We were able to establish 29 pairs. Of those, ≈ 50% produced viable egg masses. The low number of viable egg masses may be due to male moths dying before mating, resulting in unfertilized eggs being laid.
We were not as successful with our diapause program in 2007 as in 2006. Seventy-three percent of the larvae placed on diet successfully diapaused and produced pupae (Table 1). In 2006, our success rate was 92%. Differences in how we initiated diapause in 2007 may have accounted for the lower success rate. In 2006, we immediately began initiating diapause after neonates were placed on diet. In 2007, we allowed larvae to feed for three days under normal insectary conditions. Doing so may have caused higher mortality or premature pupation among some larvae as they may not have initiated diapause. Female pupae weight was lower from 2007 than from 2006, but the 2007 weights were closer to what we had historically obtained in our colony. We are also unable to explain these differences.
Table 1. Summary data from seven cohorts of sugarcane borer larvae diapaused in 2007 and removed from diapause in 2008.
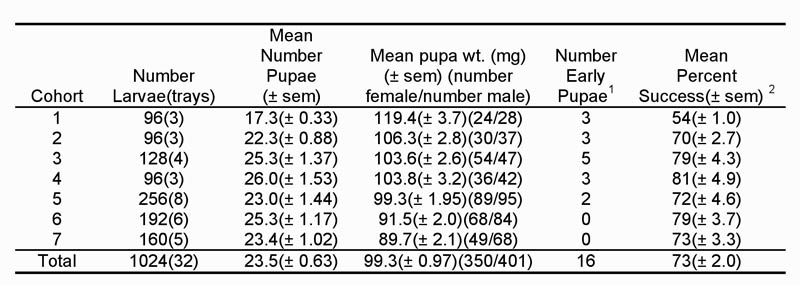
1Early pupation occurred when larvae pupated either before or when held under diapause conditions. These pupae did not produce moths.
2Percentage of larvae placed on diet and produced viable pupae.
For larvae placed in diapause in 2007, the mean number of days to pupation after termination of diapause was 16.2 days (Figure 2). Almost all pupation in 2008 occurred over a 24 day period (8 days – 32 days post termination), but an occasional larvae required > 35 days to pupate (data not shown). Percent egg hatch for 2007 averaged 84% (n = 68 egg masses; mean eggs/mass = 15). This percentage approximated historical egg data from our colony. The number of days for eggs to hatch has also remained constant. In 2008, we put our first larvae from 2007 on diet for routine rearing on 1 February. These larvae completed their life cycle within 30 days and we then were able to rebuild the colony. This allowed sufficient time to have the required numbers of larvae available for field infestations in April. Cessation of rearing resulted in a cost savings in supplies of approximately $1600.00 per annum. It also allowed our laboratory to redirect the insectary manager to another critical task in the second half of the calendar year.
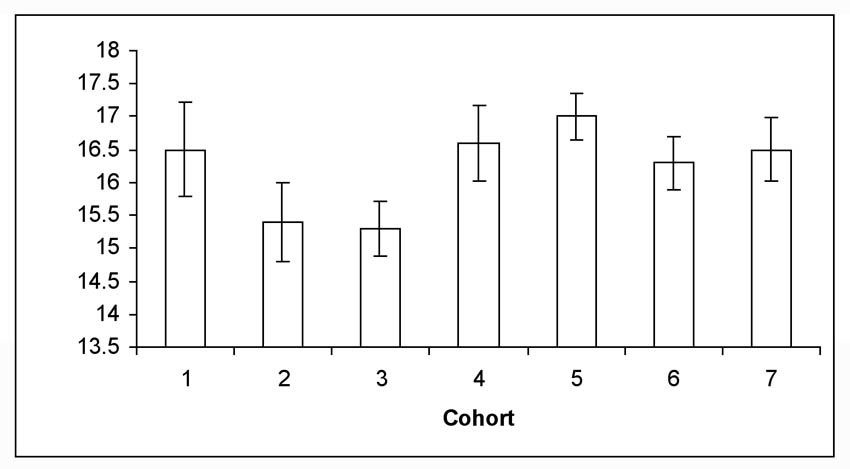
Figure 2. Mean number of days for moth emergence (± sem) from female and male pupae from seven larval cohorts placed in diapause in 2007 and removed in 2008.
In 2008, we will increase the volume of diet placed in each cell to approximately 10 ml. Some problems with the diet drying out were encountered in 2007 and increasing the volume of diet should eliminate this problem. Our procedure for inducing diapause in 2008 will be as follows: 1) divide larvae among two incubators, 2) immediately begin diapause induction, 3) induce diapause at 18°C, at photoperiod of 12:12 (L:D) h and hold for 30 days; 4) finally, a second transfer to 10° C, a photoperiod of 12:12 (L:D) h until termination of diapause. We also plan to terminate diapause on multiple dates. This will allow us to spread egg laying from our founder individuals over an extended number of days.
We have also investigated procedures to diapause a parasitoid of the sugarcane borer. This parasitoid, Cotesia flavipes Cameron (Hymenoptera: Braconidae), to our knowledge, has not been shown to diapause with its host, although earlier field work on establishing this parasite in Louisiana suggested that it could (White et al. 2004). We were able to induce diapause in this parasitoid, but our success rate was quite low (2%; n = 288 larvae stung). Our plans are to develop procedures to effectively diapause and hold this potentially important beneficial insect as well.
Acknowledgments
The author expresses appreciation to E. Duet (Biological Science Technician) of our laboratory whose hard work and dedication allowed us to develop this procedure. Appreciation is also expressed to R. Richard and J. Adams for there assistance with the colony. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
References
Davis, F. M. 1976. Production and handling of eggs of the southwestern corn borer for host plant resistance studies. Miss. Agric. For. Exp. Stn. Tech. Bull. 84.
Davis, F. M. 1983. Simple technique for storing diapausing southwestern corn borers (Lepidoptera: Pyralidae). J. Econ. Entomol. 76: 1191-119.
Davis, F. M., S. Malone, T. G. Oswalt, and W. C. Jordan. 1990. Medium sized lepidopterous rearing using multicellular rearing trays. J. Econ. Entomol. 83: 1535-1540.
Fuchs, T. W., J. A. Harding, and J. W. Smith, Jr. 1979. Induction and termination of diapause in the sugarcane borer. Ann. Entomol. Soc. Am. 72:271-274.
Hensley, S. D. 1960. A comparative study of the immature stages of three species of the Diatraea complex. Oklahoma State University, Stillwater, OK.
Katiyar, K. P., and W. H. Long. 1961. Diapause in the sugarcane borer, Diatraea saccharalis. J. Econ. Entomol. 54: 285-287.
Singh, P. 1994. History and practice of insect rearing. Shaspha 1: 17-24.
White, W. H., T. E. Reagan, J. W. Smith, Jr., and J. A. Salazar. 2004. Refuge releases of Cotesia flavipes (Hymenoptera: Braconidae) into the Louisiana sugarcane ecosystem. J. Econ. Entomol. 33: 628-632.
